Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocomposite Films
2.3. Structural Characterization of Films
2.4. Determination of the Physical Properties of Films
2.4.1. Thickness, Moisture Content (MC), Water Solubility (WS) and Swelling Degree (SD)
2.4.2. Water Vapor Permeability (WVP)
2.4.3. Optical Property
2.4.4. Mechanical Properties
2.5. Antioxidant Properties
2.6. Oxidative Stability of Soybean Oil in Film Pouches
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization of Films
3.1.1. Morphology
3.1.2. FT-IR Spectra
3.1.3. XRD Patterns
3.2. Physical Properties of Films
3.2.1. Thickness, MC, WS and SD
3.2.2. WVP
3.2.3. Optical Property
3.2.4. Mechanical Properties
3.3. Antioxidant Properties
3.4. Oxidative Stability of Soybean Oil in Film Pouches
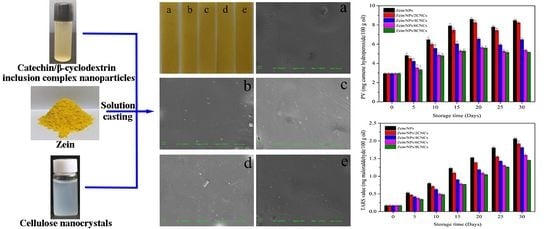
3.4.1. PV
3.4.2. TBARS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olmo, J.A.D.; Perez-Alvarez, L.; Hernaez, E.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial multilayer of chitosan and (2-carboxyethyl)-beta-cyclodextrin onto polylactic acid (PLLA). Food Hydrocoll. 2019, 88, 228–236. [Google Scholar] [CrossRef]
- Zhang, N.; Bi, F.; Xu, F.; Yong, H.; Bao, Y.; Jin, C.; Liu, J. Structure and functional properties of active packaging films prepared by incorporating different flavonols into chitosan based matrix. Int. J. Biol. Macromol. 2020, 165, 625–634. [Google Scholar] [CrossRef]
- Gao, P.; Wang, F.; Gu, F.; Ning, J.; Liang, J.; Li, N.; Ludescher, R.D. Preparation and characterization of zein thermo-modified starch films. Carbohydr. Polym. 2017, 157, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Huang, Y.; Yu, H.; Lee, T.-C.; Huang, Q. Reducing the Brittleness of Zein Films through Chemical Modification. J. Agric. Food Chem. 2011, 59, 56–61. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, B.J.; Weng, Y.M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.Y.; Ji, H.; Ran, Y.; Li, S.H.; Chen, Y. Surface modification via atmospheric cold plasma (ACP): Improved functional properties and characterization of zein film. Ind. Crop. Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Kaur, M.; Santhiya, D. UV-shielding antimicrobial zein films blended with essential oils for active food packaging. J. Appl. Polym. Sci. 2021, 138, 49832. [Google Scholar] [CrossRef]
- Shi, K.; Kokini, J.L.; Huang, Q. Engineering Zein Films with Controlled Surface Morphology and Hydrophilicity. J. Agric. Food Chem. 2009, 57, 2186–2192. [Google Scholar] [CrossRef]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M.T. Inclusion complex of clove oil with chitosan/β-cyclodextrin citrate/oxidized nanocellulose biocomposite for active food packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Ho, S.Y.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion complexation of catechin by beta-cyclodextrins: Characterization and storage stability. LWT Food Sci. Technol. 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.P.; Sun, B.G.; Wang, C.T. Physicochemical and release characterisation of garlic oil-beta-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/beta-cyclodextrin inclusion complex obtained via co-precipitation. Cyta J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, physicochemical characterization and release behavior of the inclusion complex of trans-anethole and beta-cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and characterization of zein edible films incorporated with catechin/β-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, E.; Nagy, S. A comparative study on cellulose nanocrystals extracted from bleached cotton and flax and used for casting films with glycerol and sorbitol plasticisers. Carbohydr. Polym. 2017, 174, 740–749. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- Alves, J.S.; dos Reis, K.C.; Menezes, E.G.T.; Pereira, F.V.; Pereira, J. Effect of cellulose nanocrystals and gelatin in corn starch plasticized films. Carbohydr. Polym. 2015, 115, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Lv, M.; Anderson, D.P.; Chang, P.R. Natural polysaccharide composites based on modified cellulose spheres and plasticized chitosan matrix. Food Hydrocoll. 2017, 66, 276–285. [Google Scholar] [CrossRef]
- Tang, L.R.; Huang, B.; Lu, Q.L.; Wang, S.Q.; Ou, W.; Lin, W.Y.; Chen, X.R. Ultrasonication-assisted manufacture of cellulose nanocrystals esterified with acetic acid. Bioresour. Technol. 2013, 127, 100–105. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Kolehmainen, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O.E.O. Biocomposite cellulose-alginate films: Promising packaging materials. Food Chem. 2014, 151, 343–351. [Google Scholar] [CrossRef]
- Jiang, L.W.; Yang, J.D.; Wang, Q.; Ren, L.L.; Zhou, J. Fabrication and characterisation of cellulose nanocrystals from microcrystalline cellulose by esterification and ultrasound treatment. Micro Nano Lett. 2018, 13, 1574–1579. [Google Scholar] [CrossRef]
- Mayachiew, P.; Devahastin, S. Effects of drying methods and conditions on release characteristics of edible chitosan films enriched with Indian gooseberry extract. Food Chem. 2010, 118, 594–601. [Google Scholar] [CrossRef]
- Wu, J.L.; Sun, X.Y.; Guo, X.B.; Ji, M.Y.; Wang, J.H.; Cheng, C.; Chen, L.; Wen, C.L.; Zhang, Q.Q. Physicochemical, Antioxidant, In Vitro Release, and Heat Sealing Properties of Fish Gelatin Films Incorporated with beta-Cyclodextrin/Curcumin Complexes for Apple Juice Preservation. Food Bioprocess. Technol. 2018, 11, 447–461. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yong, H.; Qin, Y.; Liu, J.; Jin, C. Development of antioxidant and antimicrobial packaging films based on chitosan and mangosteen (Garcinia mangostana L.) rind powder. Int. J. Biol. Macromol. 2020, 145, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, G. Synergistic Effect of Oleic Acid and Glycerol on Zein Film Plasticization. J. Agric. Food Chem. 2012, 60, 10075–10081. [Google Scholar] [CrossRef] [PubMed]
- Almutawah, A.; Barker, S.A.; Belton, P.S. Hydration of gluten: A dielectric, calorimetric, and fourier transform infrared study. Biomacromolecules 2007, 8, 1601–1606. [Google Scholar] [CrossRef]
- Ye, Y.; Zhu, M.; Miao, K.; Li, X.; Li, D.; Mu, C. Development of Antimicrobial Gelatin-Based Edible Films by Incorporation of Trans-Anethole/β-Cyclodextrin Inclusion Complex. Food Bioprocess. Technol. 2017, 10, 1844–1853. [Google Scholar] [CrossRef]
- Sharmin, N.; Khan, R.A.; Salmieri, S.; Dussault, D.; Bouchard, J.; Lacroix, M. Modification and Characterization of Biodegradable Methylcellulose Films with Trimethylolpropane Trimethacrylate (TMPTMA) by γ Radiation: Effect of Nanocrystalline Cellulose. J. Agric. Food Chem. 2012, 60, 623–629. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Rhim, J.-W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef]
- Sánchez-García, M.D.; Hilliou, L.; Lagarón, J.M. Morphology and Water Barrier Properties of Nanobiocomposites of κ/ι-Hybrid Carrageenan and Cellulose Nanowhiskers. J. Agric. Food Chem. 2010, 58, 12847–12857. [Google Scholar] [CrossRef]
- Perez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible Films from Carrageenan/Orange Essential Oil/Trehalose—Structure, Optical Properties, and Antimicrobial Activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.; Armada, M. Water barrier properties of starch-clay nanocomposite films. Braz. J. Food Technol. 2012. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Zheng, P.; Yu, J.; Ma, X. Preparation and properties of glycerol plasticized-starch (GPS)/cellulose nanoparticle (CN) composites. Carbohydr. Polym. 2010, 79, 301–305. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and Antioxidant Activity of the Complex of Tea Polyphenols and Oat β-Glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T.; de la Caba, K. Fish gelatin monolayer and bilayer films incorporated with epigallocatechin gallate: Properties and their use as pouches for storage of chicken skin oil. Food Hydrocoll. 2019, 89, 783–791. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- de Abreu, D.A.P.; Losada, P.P.; Maroto, J.; Cruz, J.M. Natural antioxidant active packaging film and its effect on lipid damage in frozen blue shark (Prionace glauca). Innov. Food Sci. Emerg. Technol. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Jacobsen, C. 5-Oxidation of fish oils and foods enriched with omega-3 polyunsaturated fatty acids. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E.A., Elias, R.J., Julian McClements, D., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 156–180. [Google Scholar]
- Sae-leaw, T.; Benjakul, S. Lipids from visceral depot fat of Asian seabass (Lates calcarifer): Compositions and storage stability as affected by extraction methods. Eur. J. Lipid Sci. Technol. 2017, 119, 1700198. [Google Scholar] [CrossRef]






| Films | Thickness (mm) | MC (%) | WS (%) | SD (%) |
|---|---|---|---|---|
| Zein/NPs | 0.132 ± 0.002 e | 21.82 ± 0.49 a | 14.46 ± 0.34 a | 15.7 ± 0.74 a |
| Zein/NPs/2CNCs | 0.139 ± 0.003 d | 18.62 ± 2.01 b | 12.39 ±0.22 b | 15.21 ± 1.53 a |
| Zein/NPs/4CNCs | 0.151 ± 0.001 c | 17 ± 1.29 cd | 12.19 ± 0.46 b | 13.5 ± 1 ab |
| Zein/NPs/6CNCs | 0.16 ± 0.002 b | 16.35 ± 0.11 d | 11.75 ± 0.78 b | 11.87 ± 1.24 b |
| Zein/NPs/8CNCs | 0.166 ± 0.002 a | 15.73 ± 0.26 e | 10.31 ± 0.17 c | 8.25 ± 1.62 c |
| Films | WVP (×10−7 g m−1 h−1 Pa−1) | TS (Mpa) | EAB (%) | Opacity |
|---|---|---|---|---|
| Zein/NPs | 3.27 ± 0.07 a | 12.66 ± 0.33 d | 4.5 ± 0.16 c | 5.954 ± 0.036 a |
| Zein/NPs/2CNCs | 2.63 ± 0.05 b | 22.64 ± 1.77 c | 4.76 ± 0.11 b | 5.881 ± 0.029 b |
| Zein/NPs/4CNCs | 2.2 ± 0.12 c | 29.47 ± 1.95 b | 5.2 ± 0.1 a | 4.97 ± 0.031 c |
| Zein/NPs/6CNCs | 1.75 ± 0.11 cd | 37.82 ± 1.07 a | 4.6 ± 0.16 bc | 4.668 ± 0.043 d |
| Zein/NPs/8CNCs | 1.29 ± 0.11 d | 31.14 ± 1.24 b | 4.16 ± 0.11 d | 3.096 ± 0.035 e |
| DPPH Radical Scavenging Activity (%) | Total Antioxidant Activity (%) | |||
|---|---|---|---|---|
| Storage Time (Days) | ||||
| Films | 3 | 90 | 3 | 90 |
| Zein/NPs | 87.41 ± 1.18 a | 59.7 ± 2.4 d | 91.5 ± 0.85 a | 80.04 ± 2.22 a |
| Zein/NPs/2CNCs | 87.6 ± 1.73 a | 61.39 ± 1.07 cd | 91.64 ± 1.37 a | 81.1 ± 2.02 a |
| Zein/NPs/4CNCs | 87.23 ± 1.28 a | 64.43 ± 1.34 bc | 92.19 ± 2.24 a | 81.9 ± 1.91 a |
| Zein/NPs/6CNCs | 87.9 ± 1.61 a | 67.47 ± 2.4 ab | 91.96 ± 2.13 a | 82.86 ± 2.56 a |
| Zein/NPs/8CNCs | 87.35 ± 2.72 a | 68.94 ± 2.33 a | 92.1 ± 1.99 a | 83.35 ± 3.83 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Han, Y.; Meng, X.; Xiao, Y.; Zhang, H. Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging. Polymers 2021, 13, 2759. https://doi.org/10.3390/polym13162759
Jiang L, Han Y, Meng X, Xiao Y, Zhang H. Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging. Polymers. 2021; 13(16):2759. https://doi.org/10.3390/polym13162759
Chicago/Turabian StyleJiang, Longwei, Yanlong Han, Xiangyi Meng, Yawen Xiao, and Huajiang Zhang. 2021. "Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging" Polymers 13, no. 16: 2759. https://doi.org/10.3390/polym13162759
APA StyleJiang, L., Han, Y., Meng, X., Xiao, Y., & Zhang, H. (2021). Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging. Polymers, 13(16), 2759. https://doi.org/10.3390/polym13162759