1. Introduction
Steel is used in many areas as a major material in building construction, offshore structure, ships, bridges and airports. Despite its exceptional strength, steel is normally not able to withstand high temperatures between 470 and 500 °C, causing it to lose its strength slowly [
1]. In building structures, failure of the load-bearing members of steel structures will lead to parts’ disintegration. Due to the presence of hydrocarbon and natural gas, a gas-pipe explosion in a building can occur if the pipe ruptures. For safety reasons, fire-retardant materials are essentially used as shields or coating to maintain the temperature of steel structures below 500 °C, in case of fire incidents [
2].
There are two commonly used materials in steel coatings, namely cementitious materials and intumescent coating. The former is usually inorganic, not combustible when in contact with fire, durable and inexpensive. However, the coating from these materials has to be thick and thus too heavy to provide sufficient protection on the substrate. Intumescent coating, such as sodium silicate–based paint, is normally light, aesthetic and smooth and can be applied thinly. Its only drawbacks are poor water resistance and the tendency to form its original constituents, which are sodium carbonate and silicon dioxide, when reacting with carbon dioxide [
3].
The intumescent coating expands when exposed to heat and creates a char layer to protect the steel substrate. Intumescence occurs from the interaction of three components: (i) an inorganic acid, such as ammonium polyphosphate (APP); (ii) a carbonaceous char-forming material, such as polyols; and (iii) a blowing agent, such as melamine [
4]. Appropriate composition in making intumescent coating is crucial in producing the best performance for the intumescent system. Geopolymer has the potential as an intumescent coating [
5,
6,
7,
8].
To make the composites more environmentally friendly, the use of SiO
2-rich materials as alternative is necessary. Abundant agricultural wastes containing high content of SiO
2, such as rice husk ash (RHA), are viewed as cleaner and greener substitutes for improving the performance of geopolymeric composites. Most of the world’s rice is produced in Asia, and Asians account for more than 80 percent of global rice consumption. Rice production is estimated to be 156 million tons per year, resulting in the rice industry generating massive amount of solid waste [
9]. RHA is a by-product obtained by burning rice husk for several heating purposes. RHA burned at 600 °C for 2 h, yielded a light grey husk [
10]. Burning temperatures below 700 °C can be used to produce amorphous silica, which has a large surface area. When burning with oxygen at temperatures lower than 500 °C for an extended period, amorphous silica can be produced as well [
11]. It comprises mostly the reactive amorphous SiO
2 phase, depending on the burning process [
12]. It is available and suitable for silicate polymeric condensation [
13]. In building sector, geopolymer concrete led to reduced carbon dioxide emission compared to the ordinary Portland cement (OPC) mortar. Thus, it helps decrease the pollution problems created through its disposal in landfills, especially in important rice-producing countries such as in Thailand and India.
Geopolymer is an amorphous solid inorganic polymer with a tri-dimensional aluminosilicate structure produced through geopolymerization, the chemical reaction between alkaline solution and aluminosilicate source [
14]. Biomass wastes, such as rice husk ash and palm oil fuel ash, have been recycled as energy sources [
6,
15]. Other common aluminosilicate sources include metakaolin, fly ash and ground granulated blast-furnace slag (GBBS) [
16,
17,
18]. Geopolymer using an aluminosilicate source has shown excellent fire-retardant properties. In 1970, Davidovits [
19] developed a geopolymer material as a fire-resistant system in buildings following a tragic fire which occurred in Europe. Later in 2008, a study was conducted on the fire performance of three different types of geopolymer, namely Na-poly(sialate), K-poly(sialate-siloxo) and K-poly(sialate-disiloxo) with a Si/Al ratio of 1, 2 and 3 respectively [
20]. The fire-resistant test was conducted by using a direct flame at 1000 °C, on a 10 mm thick geopolymer composite panel. The non-exposed surface temperature was subsequently measured after 30 min. Exposed surface refers to the surface that is directly in contact with the flame. The surface at the back of the geopolymer composite panel is referred to as the non-exposed surface.
Rickard et al. [
21] investigated the thermal expansion and thermal stability of five different types of fly ash (FA) in order to find out the potential of the geopolymer paste activated by sodium aluminate as a thermal barrier material. The composition of the amorphous component of each fly ash affected by the thermal test was determined by conducting XRF and XRF tests. The combined results showed that the thermal performance of geopolymer was strongly influenced by the Si/Al ratio. Si/Al ratio of higher than five produced geopolymers with excellent dimensional stability during heating at 1000 °C, while those with ratios lower than two (2) showed poor thermal stability. At high temperatures between 500 and 850 °C, sharp shrinkage occurred in all samples due to the compaction and densification of the samples by heat and the continuous steady motion of the aluminosilicate particles.
For lightweight panel applications, Abdul Rashid et al. [
22] investigated the fire resistance of composite coatings reinforced with biofillers derived from geopolymer materials. The coating formulation was optimized in terms of thickness, alkaline activator ratio and curing regime, resulting in a RHA-based geopolymer that remained intact for nearly two hours when exposed to flame for a duration of one hour and forty minutes. They concluded that a geopolymer-binder-type composite is a plausible fire-resistant coating for structural insulated panels.
Sarazin et al. [
7] investigated the fire resistance of novel geopolymer (GP) foams based on alkali-activated metakaolin and silica fume (SF). Fresh GP foams were applied as coatings on steel plates. After one week of curing, the foams were subjected to a flame burn-through test. Results showed that GP foams are excellent thermal barriers which recorded a temperature difference of 251 °C when compared to the uncoated steel plates. Le and Louda [
23] studied the effect of time and temperature on the strengths and fire resistance of geopolymer foam (GF) coated on an aluminum plate. The composition of the GF used in the experiment included a potassium activator, basalt ground fiber and aluminum powder with a mass ratio to the binder of 0.45, 0.3 and 0.15, respectively. The results showed that the GF exhibited a substantial increase after three days in its compressive strength by 122.9% at the optimal temperature of 85 °C for two hours.
Geopolymer has shown to have excellent fire-retardant properties in studies using aluminosilicate sources. However, research on its fire-retardant properties and thermal properties of geopolymer coating using RHA and statistical analysis tools is rather limited. Studies conducted to identify the impact of several factors on the properties of the RHA-based geopolymer coating is also still minimal. In addition, recent studies have not adopted the improved method in determining the optimum formulation through statistical optimization.
Despite many publications on rice-husk-ash-based polymer composite reported in the literature in recent years [
5,
8,
24,
25,
26], most studies were carried out on the one-factor-a-time (OFAT) approach. The application of statistical analysis and regression coefficients (mathematical models) is required effectively identify the optimal composition of geopolymer coating with improved fire-retardant and thermal properties to increase efficiency. The design of experiment (DOE) offers numerous advantages over the OFAT approach, including low resource requirements (experimental runs, time, materials and human resources), accurate measurement of main effects and interactions and the ability to simultaneously analyze multiple variables [
27]. Furthermore, the RSM, initially coined by Box and Wilson [
28], is widely used as a mathematical model for investigating significant effects, interactions and optimization conditions. The central composite design (CCD) is the most effective model for analysis and design [
29].
Table 1 shows the difference in the total number of experimental runs between the full design of RSM and the full factorial (classical method) design based on 5-level factors. The former requires only 31 experimental runs (with one replication) for analyzing four factors, whereas a full factorial design requires 625 experimental runs to arrive at a comparable result [
24].
Since the RSM approach, specifically the CDD, has been widely used in polymer optimization [
30,
31,
32,
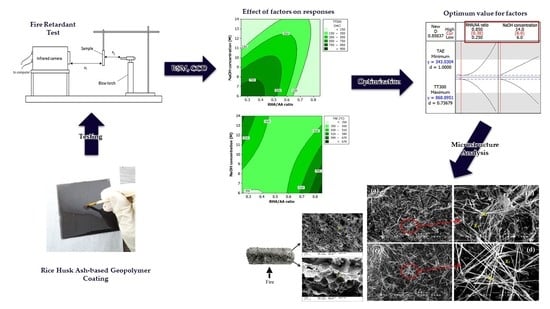
33], it was thus adopted in this study. The objectives of this paper are to identify the significant effect of different factors (RHA/AA ratio and NaOH concentration) on the fire-retardant and thermal properties of RHA-based geopolymer coating, to determine the optimum composition for the RHA-based geopolymer coating and to study the microstructure and element characteristics of the RHA-based geopolymer coating.
2. Materials and Methods
2.1. Factors and Levels of the Design of Experiment (DOE)
In the study, the ratio of rice husk ash/activated alkaline solution (RHA/AA) and the sodium hydroxide (NaOH) concentration, designated as V
1 and V
2, respectively, were chosen as factors. Based on the preliminary results using a screening process with fractional factorial design (FrFD), other factors, such as the ratio of AA solution, curing temperature and curing time, were kept constant at 5.5, 70 °C and seven days, respectively. Factors and levels used in the DOE are shown in
Table 2.
2.2. Design of Experiment
At each design stage, five levels and two factors were applied in the CCD and with two replications for a total of 26 experimental runs. The factors were selected based on preliminary lab work, their significant effect on the responses and their working range (workability).
Table 3 displays the complete CCD with coded and uncoded levels of these factors. The value for the total block is 1, with the experiments carried out in randomized order.
The optimization of RHA-based geopolymer coating was conducted by using Minitab @ 16.2 (Minitab, LLC, State College, PA, USA). An analysis of variance (ANOVA) was used to calculate the significance of the main factors and their interactions. The value of 95% was set as the significance level, which reflected the
p-value of 0.05. Based on the correlation coefficient (R
2) value, the regression coefficient model (mathematical model) developed in the ANOVA table was used for optimization. To acquire the regression coefficient model, experimental data were fitted with the second-order polynomial model. The general mathematical model obtained from the analysis is shown in Equation (1),
where Υ is the response; β
0, β
i, β
ii and β
ij are regression coefficients for the intercept, linear, quadratic and interaction terms, respectively; and X
i and X
j are coded values for the independent variables [
34].
2.3. Raw Materials and Sample Preparation
RHA was obtained from Maero Tech Sdn. Bhd (Nilai, Malaysia). Rice husk was burnt at a temperature of approximately 600 °C for 24 h to produce RHA. The material was ground, using a planetary mill (Pulverisette 4, FRITSCH GmbH—Milling and Sizing, Idar-Oberstein, Germany), and sieved through 75 micron opening to obtain finer particle sizes.
Figure 1 shows images of RHA before and after grinding.
The fine structure of RHA before and after grinding was viewed under a S-3400N scanning electron microscope (SEM) (Hitachi, Tokyo, Japan). RHA particle size before grinding ranges from 1 to 100 μm. Particles appear as plates and thin shell-like structures with rectangular indents on the surface. These forms constitute the initial structure of the RH. RHA has porous, cellular surfaces due to its sponge-like character.
Figure 2 shows the RHA structure after grinding. The particles contain high silica content with amorphous shapes similar to cristobalite and trace crystalline quartz [
35].
In order to develop pozzolanic activity, RHA was ground to a very fine particle size [
36]. The condition for burning RH is vital in producing the highest silica RHA in an amorphous state. Conversely, silica derived from unchecked incineration (temperatures higher than 700 to 800 °C) comprises mainly cristobalite and tridymite, which are non-reactive silica minerals [
37]. The physical properties of the RHA after grinding are given in
Table 4. The color of RHA was determined visually, and a hexadecimal (HEX) color code was generated by using a vector graphics editor and design program (Adobe Illustrator, Adobe, Inc., San Jose, CA, USA). A HEX color code of #d3d3d3 was determined, indicating that the color is light gray.
Sodium hydroxide (NaOH) and sodium silicate (Na
2SiO
3) were purchased from Evergreen Engineering & Resources (Semenyih, Malaysia). The sodium-based solution was chosen over potassium-based, due to lower material cost [
38] and better mechanical properties as reported in previous studies [
39]. The sodium silicate solution was purchased from LGC Scientific Sdn Bhd (Selangor, Malaysia). The chemical composition (by wt.%) of the solution was Na
2O = 11.9%, SiO
2 = 57.8% and H
2O = 30.3%. Sodium hydroxide pellets with 97% purity were provided by Merck KGaA (Darmstadt, Germany). Different concentrations expressed as molarity, M of NaOH solution were prepared based on the amount of pellets dissolved in de-ionized water.
Mild steel plates with a thickness of 1 ± 0.05 mm and dimensions of 100 mm in length and 100 mm in width were cleaned by using sandpaper to improve the surface roughness and then washed with acetone to remove any unwanted oils or greases. Once the surface was dried at room temperature, the plates were placed in an oven at 45 °C for further drying to remove excess water. These were then used as substrate and coated with rice-husk-ash-based geopolymer coating.
Samples were prepared according to the flowchart, as shown in
Figure 3. Na
2SiO
3 was added into NaOH solution at a ratio of 5.5 to form an activated alkali solution (AA) solution. The solution was then mixed with RHA at a designated ratio (
Table 3) to obtain a dark gray slurry mixture. The mixture was then stirred with a mechanical stirrer (HS-300, WiseStir, Thessaloniki, Greece) at 150 rpm for 30 min, until homogenous. The mixture was strained through a small sieve directly onto the mild steel plate at 0.3 g/cm
2 and was spread evenly, as shown in
Figure 4.
The coated substrate was left for one minute for the coating binder to self-level. It was then placed in a vacuum oven (Model 53, Binder, Tuttlingen, Germany) for degasification to remove the remaining tiny bubbles. The coated substrate was pre-dried by placing it in an oven for approximately 40 min at 40 °C. The coated substrate was then removed from the oven and pressed by using a press machine (Gotech, Taichung City, Taiwan) to obtain the desired thickness. The thickness of the steel plate was 2 mm, which equaled the total thickness of the geopolymer binder coating (1 mm) and mild steel substrate (1 mm). The thickness was measured by using a digital Vernier caliper up to two decimal points accuracy. Then, the excess coating binder was trimmed, and the coated substrate was placed again in the oven to cure for 24 h at 50 °C. After 24 h, the coated substrate was left at room temperature for six days or more (depending on the experimental design) for complete curing. While geopolymer binder typically cures and hardens in 4 to 24 h, a longer curing duration was opted to ensure that the coatings were fully hardened and stabilized in ambient conditions.
2.4. Fire-Retardant Test
The test was conducted by heating coated samples with direct blow torch flame following the UL-1709 standards [
40]. The samples were heated directly, using a blow torch with flame temperatures around 900 °C. The specimens were kept 60 cm apart from the infrared camera (X
1) and 7 cm from the blow torch (X
2), as shown in
Figure 5. The distance was determined based on previous literature and the safety of the infrared camera [
41,
42]. A shorter distance between infrared camera and specimens will result in camera overheat and damage. The distance over 7 cm will result in the blue flame not being in contact with the specimens, while the distance below 7 cm will result in the coating not having enough space to intumescent. At the beginning of every test, X
1, X
2, the ambient temperature and humidity were recorded on the computer. The recorded parameters were relevant since radiation was absorbed in the ambient space and transmittance was reduced with distance. Flame temperature versus time was taken as a result of fire protection in the experiment. A bare mild steel plate which acted as a control for the purpose of comparison was first exposed to direct flame for 10 min. A coated mild steel plate was exposed to direct flame for at least 20 min or until equilibrium temperature was reached.
2.5. Microstructure of Rice Husk Ash
A scanning electron microscope (SEM) (Hitachi, Tokyo, Japan) was used to analyze the difference in microstructure of samples before and after testing. SEM was conducted by using Hitachi S-3400N variable SEM. A total of five samples were taken, with two samples before the fire-retardant test, two samples after the test and one intumescent coating sample. The samples were first mounted with a conductive adhesive and sputter-coated with gold-palladium powder. The stub with sample specimens was inserted into the sample chamber of the SEM for viewing. Micrographs of sample surface were taken at magnifications of 100×, 200× and 1000×. Samples were also analyzed, using EDX, which is attached in the SEM, to determine elements in the specimens, including oxygen, silica, sodium, iron, carbon and calcium.
2.6. Thermogravimetry Analysis (TGA)
TGA is a method for measuring the weight loss of a material due to either increase in temperature or time. TGA was carried out by using SDTA 850 Mettler Toledo micro and ultra-micro balances in an atmosphere of flowing nitrogen gas in alumina crucible, at a heating rate of 10 °C/min over a temperature range from 50 to 1000 °C. Specimens, in the form of powder (RHA) and fine solid (geopolymer binder), weighing approximately 10 mg, were placed in crucibles.
2.7. X-ray Diffraction (XRD)
XRD was used to study the crystal structures of a material. Geopolymer residue samples and RHA were packed in circular cavity holders, and the phase composition of the samples was determined by using an Ital Structure APD 2000 diffractometer. Using a copper cathode in the 2θ angle range from 5° to 80°, the machine was operated with back monochrome running at 40 kW and 30 mA. The peak search for minerals in the diffraction data was conducted by using the PANalytical X’Pert HighScore software (version 1.0d). The software compared the d-spacing and intensity of each mineral with those of reference mineral standards.
2.8. Differential Scanning Calorimetric (DSC)
DSC measurements of geopolymer and RHA were conducted by using a Mettler Toledo DSC 823 with aluminum crucibles containing 5 to 10 mg of samples for the purpose of studying thermal transition following changes in a material, such as polymer, when it heated. The test was made under a dynamic nitrogen atmosphere at 60 mL/min and a heating rate of 5 °C/min in the temperature range of −30 to 160 °C. The DSC cell was calibrated with indium since it could identify heat of fusion and melting point efficiently. An empty pan sealed with a cover pan was used as a reference pan.
2.9. Coefficient of Thermal Expansion (CTE)
A dilatometer was used to monitor the CTE of the geopolymer sample expands or shrinks on heating. The test was conducted by using a DI-24 ADAMEL LHOMARGY dilatometer. Before testing, the samples were cast into a 1 mL syringe and cut to length to achieve a cylinder of 7.5 mm in diameter and 5 mm in length. The geopolymer samples were then positioned between two spacers, each measuring 5.0 mm in diameter and 0.7 mm thick. The alumina pushrod (piston) pushed the sample to position it next to the stationary alumina key. Once prepared, the sample holder was inserted into the interior of the combustion tube in the furnace and sealed by a high vacuum fitting. Measurement was conducted according to ASTM E831 standards between the temperature of 0 °C and 850 °C with a heating rate of 5 °C per minute.