Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymers
2.3. Analytical and Testing Methods
3. Results and Discussion
3.1. Phase Composition
3.2. Thermal Properties
3.2.1. Thermal Dilatometry
3.2.2. Pyrometric Cone Refractoriness
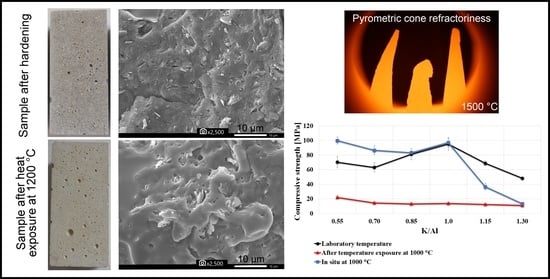
3.3. Mechanical Properties
3.4. Porosity
3.5. Morphology
4. Conclusions
- The X-ray diffractograms showed that geopolymers had a stable amorphous phase up to temperatures of 800–1200 °C depending on the K/Al ratio. The temperature of formation of the crystalline phases decreased with increasing K/Al ratios.
- Increasing of K/Al ratio led to a decline in the onset temperature of the major shrinkage. The addition of chamotte into geopolymer binders resulted in a decrease of total shrinkage of the geopolymer composites (from 12–20% to 0.4–2.1%).
- Increasing addition of potassium content led to a rise in the refractoriness of geopolymer binders. The addition of chamotte to geopolymer binders resulted in a reduction of their refractoriness.
- The compressive and flexural strength and modulus of elasticity decreased with growing K/Al ratios. The exception was the local maximum at K/Al ratio 1, which was the most evident at laboratory temperature and at 200 °C.
- Results of the compressive strength tested in situ did not show comparable results with compressive strength tested after exposure to elevated temperatures, which followed a decreasing path with increasing temperature. The values of compressive strength tested in situ began to rise from 600 °C.
- Average pore diameter increased slightly with increasing potassium content at laboratory temperature and at elevated temperatures up to 800 °C, while from 1000 °C to 1200 °C, the pore size drastically decreased. The effect of K/Al ratio on total pore volume was not significant.
- As a result of calcination at high temperatures, phase changes occurred in the binder and the inhomogeneous geopolymer matrix with undissolved metakaolinite plates turning into a homogeneous porous structure with visible pores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rovnanik, P.; Safrankova, K. Thermal Behaviour of Metakaolin/Fly Ash Geopolymers with Chamotte Aggregate. Materials 2016, 9, 535. [Google Scholar] [CrossRef]
- Perná, I.; Novotná, M.; Řimnáčová, D.; Šupová, M. New Metakaolin-Based Geopolymers with the Addition of Different Types of Waste Stone Powder. Crystals 2021, 11, 983. [Google Scholar] [CrossRef]
- Bouna, L.; Ait El Fakir, A.; Benlhachemi, A.; Draoui, K.; Ezahri, M.; Bakiz, B.; Villain, S.; Guinneton, F.; Elalem, N. Synthesis and characterization of mesoporous geopolymer based on Moroccan kaolinite rich clay. Appl. Clay Sci. 2020, 196, 105764. [Google Scholar] [CrossRef]
- Lyon, R.E.; Balaguru, P.N.; Foden, A.; Sorathia, U.; Davidovits, J.; Davidovics, M. Fire-resistant Aluminosilicate Composites. Fire Mater. 1997, 21, 67–73. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Chemistry and Applications; Institut Géopolymère: Saint-Quentin, France, 2008; p. 285. [Google Scholar]
- Davidovits, J. Properties of geopolymer cement. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; pp. 131–149. [Google Scholar]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H. 22-Alkali-activated cements for protective coating of OPC concrete. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Oxford, UK, 2015; pp. 605–626. [Google Scholar]
- Alzeer, M.I.M.; MacKenzie, K.J.D.; Keyzers, R.A. Porous aluminosilicate inorganic polymers (geopolymers): A new class of environmentally benign heterogeneous solid acid catalysts. Appl. Catal. A Gen. 2016, 524, 173–181. [Google Scholar] [CrossRef]
- Haincova, E.; Hajkova, P.; Kohout, J. Prepregs for Temperature Resistant Composites. Materials 2019, 12, 4012. [Google Scholar] [CrossRef] [Green Version]
- Haincova, E.; Hajkova, P. Effect of Boric Acid Content in Aluminosilicate Matrix on Mechanical Properties of Carbon Prepreg Composites. Materials 2020, 13, 5409. [Google Scholar] [CrossRef] [PubMed]
- Mužek, M.N.; Svilović, S.; Zelić, J. Fly ash-based geopolymeric adsorbent for copper ion removal from wastewater. Desalination Water Treat. 2013, 52, 2519–2526. [Google Scholar] [CrossRef]
- Xia, M.; Sanjayan, J. Method of formulating geopolymer for 3D printing for construction applications. Mater. Des. 2016, 110, 382–390. [Google Scholar] [CrossRef]
- Lancellotti, I.; Barbieri, L.; Leonelli, C. 20-Use of alkali-activated concrete binders for toxic waste immobilization. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Oxford, UK, 2015; pp. 539–554. [Google Scholar]
- Kohout, J.; Koutnik, P. Effect of Filler Type on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Materials 2020, 13, 2395. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.-F.; Dai, J.-G. Understanding geopolymer binder-aggregate interfacial characteristics at molecular level. Cem. Concr. Res. 2021, 149, 106582. [Google Scholar] [CrossRef]
- Thang, N.H.; Nhung, L.T.; Quyen, P.V.T.H.; Phong, D.T.; Khe, D.T.; Van Phuc, N. Development of heat resistant geopolymer-based materials from red mud and rice husk ash. In Proceedings of the 2nd International Conference on Applied Sciences, Ho Chi Minh City, Vietnam, 24–25 May 2018; Volume 1954. [Google Scholar]
- Hájková, P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals 2018, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Koutník, P.; Soukup, A.; Bezucha, P.; Šafář, J.; Kohout, J. Low viscosity metakaolinite based geopolymer binders. Constr. Build. Mater. 2020, 230, 116978. [Google Scholar] [CrossRef]
- Kohout, J.; Koutník, P.; Bezucha, P.; Kwoczynski, Z. Leachability of the metakaolinite-rich materials in different alkaline solutions. Mater. Today Commun. 2019, 21, 100669. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Yan, D.; Xie, L.; Qian, X.; Ruan, S.; Zeng, Q. Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials 2020, 13, 2973. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Chinje Melo, U.F.; Delplancke, M.-P.; Rahier, H. Influence of the activating solution composition on the stability and thermo-mechanical properties of inorganic polymers (geopolymers) from volcanic ash. Constr. Build. Mater. 2013, 48, 278–286. [Google Scholar] [CrossRef]
- Vitola, L.; Pundiene, I.; Pranckeviciene, J.; Bajare, D. The Impact of the Amount of Water Used in Activation Solution and the Initial Temperature of Paste on the Rheological Behaviour and Structural Evolution of Metakaolin-Based Geopolymer Pastes. Sustainability 2020, 12, 8216. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.-H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Silva, P.D.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.-y. Effect of Na/Al on formation, structures and properties of metakaolin based Na-geopolymer. Constr. Build. Mater. 2019, 226, 250–258. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Lu, Y.; Bai, X. Effects of Na/Al ratio on mechanical properties and microstructure of red mud-coal metakaolin geopolymer. Constr. Build. Mater. 2020, 263, 120653. [Google Scholar] [CrossRef]
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.-H. Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram. Int. 2017, 43, 11433–11441. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Tawfik, A.; Abd El-Raoof, F.; Katsuki, H.; MacKenzie, K.J.D.; Komarneni, S. K-Based Geopolymer from metakaolin: Roles of K/Al ratio and water or steam Curing at different temperatures. Mater. Constr. 2016, 66, e081. [Google Scholar] [CrossRef] [Green Version]
- Koutnik, P. Comparison of Kaolin and Kaolinitic Claystones as Raw Materials for Preparing Meta-Kaolinite-Based Geopolymers. Ceramics–Silikaty 2019, 63, 110–123. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Synthesis and thermal behaviour of potassium sialate geopolymers. Mater. Lett. 2003, 57, 1477–1482. [Google Scholar] [CrossRef]
- Lin, T.S.; Jia, D.C.; He, P.G.; Wang, M.R. Thermo-mechanical and Microstructural Characterization of Geopolymers with α-Al2O3 Particle Filler. Int. J. Thermophys. 2009, 30, 1568–1577. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal evolution of metakaolin geopolymers: Part 1–Physical evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Medri, V.; Fabbri, S.; Ruffini, A.; Dedecek, J.; Vaccari, A. SiC-based refractory paints prepared with alkali aluminosilicate binders. J. Eur. Ceram. Soc. 2011, 31, 2155–2165. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C.; Brown, P. Ambient Temperature Drying Shrinkage and Cracking in Metakaolin-Based Geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef] [Green Version]
- Kovářík, T.; Rieger, D.; Kadlec, J.; Křenek, T.; Kullová, L.; Pola, M.; Bělský, P.; Franče, P.; Říha, J. Thermomechanical properties of particle-reinforced geopolymer composite with various aggregate gradation of fine ceramic filler. Constr. Build. Mater. 2017, 143, 599–606. [Google Scholar] [CrossRef]
- Westman, A.E.R. The Thermal Expansion of Fireclay Bricks. Univ. Ill. Bull. 1928, 26, 1–30. [Google Scholar]
- Okada, K.; Ooyama, A.; Isobe, T.; Kameshima, Y.; Nakajima, A.; MacKenzie, K.J.D. Water retention properties of porous geopolymers for use in cooling applications. J. Eur. Ceram. Soc. 2009, 29, 1917–1923. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Melo, U.F.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- Musil, S.S.; Kriven, W.M.; Biernacki, J. In SituMechanical Properties of Chamotte Particulate Reinforced, Potassium Geopolymer. J. Am. Ceram. Soc. 2014, 97, 907–915. [Google Scholar] [CrossRef]
- Trindade, A.C.C.; Silva, F.d.A.; Alcamand, H.A.; Borges, P.H.R. On The Mechanical Behavior of Metakaolin Based Geopolymers Under Elevated Temperatures. Mater. Res. 2017, 20 (Suppl. S2), 265–272. [Google Scholar] [CrossRef] [Green Version]
- Rovnaník, P. Effect of the aggregate type on the properties of alkali-activated slag subjected to high temperatures. Mater. Tehnol. 2015, 49, 709–713. [Google Scholar] [CrossRef]
- Fayyad, S.M.; Al-Marahleh, G.S.; Abu-Ein, S.Q. Improvement of the Refractoriness under Load of Fire-Clay Refractory Bricks. Adv. Theor. Appl. Mech. 2012, 5, 161–172. [Google Scholar]
- Zhang, M.; Zhao, M.; Zhang, G.; El-Korchi, T.; Tao, M. A multiscale investigation of reaction kinetics, phase formation, and mechanical properties of metakaolin geopolymers. Cem. Concr. Compos. 2017, 78, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of Ceramics from Metakaolin-Based Geopolymers. Part II: K-Based Geopolymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar] [CrossRef]












| Material | Material Composition (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a LOI | H2O | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | V2O5 | ZrO2 | |
| Mefisto L05 | 1.30 | - | 52.2 | 42.9 | 0.88 | 0.16 | - | - | 0.75 | 1.62 | 0.07 | 0.05 | 0.03 |
| Potassium silicate | - | 60.4 | 27.0 | 0.05 | 0.01 | - | - | 0.38 | 12.1 | - | - | - | - |
| Chamotte | 0.02 | - | 52.1 | 43.6 | 1.24 | 0.17 | 0.12 | 0.05 | 0.94 | 1.65 | 0.06 | - | 0.05 |
| Material | Specific Gravity | Bulk Density | Particle Size | Specific Surface Area (BET) | |
|---|---|---|---|---|---|
| (kg/m3) | (kg/m3) | d50 (µm) | d90 (µm) | (m2/g) | |
| Mefisto L05 | 2455 | 614 | 5.58 | 16.47 | 13.4 |
| Chamotte | 2541 | 1494 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohout, J.; Koutník, P.; Hájková, P.; Kohoutová, E.; Soukup, A. Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers 2021, 13, 3754. https://doi.org/10.3390/polym13213754
Kohout J, Koutník P, Hájková P, Kohoutová E, Soukup A. Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers. 2021; 13(21):3754. https://doi.org/10.3390/polym13213754
Chicago/Turabian StyleKohout, Jan, Petr Koutník, Pavlína Hájková, Eliška Kohoutová, and Aleš Soukup. 2021. "Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites" Polymers 13, no. 21: 3754. https://doi.org/10.3390/polym13213754
APA StyleKohout, J., Koutník, P., Hájková, P., Kohoutová, E., & Soukup, A. (2021). Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers, 13(21), 3754. https://doi.org/10.3390/polym13213754