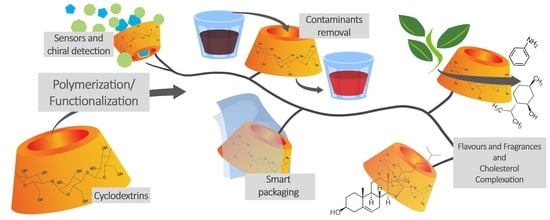
Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues
Abstract
:1. Introduction
2. Classification of CD-Based Polymers
3. Applications of Cyclodextrin-Based Materials and Polymers in Food Science
3.1. An Overview of the Use of Cyclodextrin-Based Materials to Complex Cholesterol
| Application | Material Classification | Type of Material | Biomolecule | Effect | Reference |
|---|---|---|---|---|---|
| Cholesterol extraction | CD random suspended polymer | Chitosan with immobilized β-CD | Cholesterol | Cholesterol extraction | [69] |
| Cholesterol extraction | Crosslinked CD polymer | Adipic acid with β-CD | Cholesterol | Cholesterol extraction | [70] |
| Cholesterol extraction | Crosslinked CD polymer | Epichlorohydrin and β-CD | Cholesterol | Cholesterol extraction | [71] |
| Cholesterol extraction | Crosslinked CD polymer | Cellulose and β-CD with glutaraldehyde | Cholesterol | Cholesterol extraction | [73] |
| Contaminants | Crosslinked CD polymer | Active carbon CD | Methylene blue | Contaminant removing | [79] |
| Contaminants | Crosslinked CD polymer | Cellulose nanofibrils/β-CD | Microcystin-LR/methylene blue | Contaminant removing | [80] |
| Contaminants | Crosslinked CD polymer | Cyclodextrin-based nanosponges (CD-NSs) | 2-MIB | Contaminant removing | [81] |
| Contaminants | Crosslinked CD polymer | CD-NSs | Ciprofloxacin | Contaminant removing | [82] |
| Contaminants | Crosslinked CD polymer | CDs containing amino and amido groups | PFAS | Contaminant removing | [83] |
| Contaminants | CD end-capped polymer | Amphiphilic CDs in PFS membrane | Endocrine-disrupting plasticizer | Contaminant removing | [84] |
| Contaminants | Crosslinked CD polymer | Mesoporous β-CD polymers | Heavy metals | Contaminant removing | [85] |
| Contaminants | Self-assembled supramolecular network | Insoluble β-CD bead polymers | ZEN | Contaminant removing | [86] |
| Contaminants | Self-assembled supramolecular network | Insoluble β-CD beads polymers | AOH | Contaminant removing | [87] |
| Contaminants | Crosslinked CD polymer | CD-NSs | Indole | Contaminant removing | [88] |
| Extraction | Crosslinked CD polymer | CDs crosslinked with epichlorohydrin, hexamethylene diisocyanate and phenyl isocyanate | Naringin and limonin | Extraction of undesirable molecules | [89] |
| Extraction | Crosslinked CD polymer | CM-HP-β-CDCP-MNPs | Rutin | Extraction of bioactive compound | [90] |
| Food packaging | CD-loaded polymer | β-CD + low-density polyethylene | Carvacrol/trans-cinnamaldehyde | Antimicrobial | [91] |
| Food packaging | Crosslinked CD polymer | β-CD + chitosan | Carvacrol/trans-cinnamaldehyde | Antimicrobial | [92] |
| Food packaging | Crosslinked CD polymer | β-CD + microfibrillated cellulose | Carvacrol | Antimicrobial | [93] |
| Food packaging | CD-loaded polymer | β-CD + halloysite nanotubes | Carvacrol + cinnamon + oregano essential oils | Antimicrobial | [94] |
| Food packaging | Crosslinked CD polymer | HPβ-CD + TEMPO-oxidized cellulose nanocrystals | Carvacrol/curcumin | Antimicrobial | [95] |
| Food packaging | - | Titanium dioxide nanoparticles with α-CD and β-CD | Sorbic acid/benzoic acid | Antimicrobial | [96] |
| Food packaging | Crosslinked CD polymer | α-NS, β-NS and HPβ-NS | Coriander essential oil | Antimicrobial | [97] |
| Food packaging | Crosslinked CD polymer | α-NS, β-NS and HPβ-NS | Cinnamon essential oil | Antimicrobial | [98] |
| Food packaging | CD-loaded polymer | β-CD + chitosan edible coating | trans-Cinnamaldehyde | Antimicrobial | [99] |
| Food packaging | CD-loaded polymer | β-CD + sodium alginate edible coating | trans-Cinnamaldehyde | Antimicrobial | [100] |
| Food packaging | CD-loaded polymer | α-CD + polystyrene | Ethylene | Ripening control | [101] |
| Food packaging | CD-loaded polymer | α-CD + polystyrene (electrospun nanofibers) | 1-Methylcyclopropene | Ripening control | [102] |
| Food packaging | - | γ-CD + metal–organic frameworks | Hexanal | Ripening control | [103] |
| Food packaging | CD-loaded polymer | CD + polyvinyl chloride | Capturing contaminants | [104] | |
| Food packaging | Self-assembled supramolecular network | β-CD + zein | Cholesterol | Reduction of cholesterol | [105] |
| Food packaging | CD-loaded polymer | Triacetyl β-CD + low-density polyethylene (electrospun nanofibers) | Sulfur off-flavors | Fragrant | [106] |
| Food packaging | CD-loaded polymer | β-CD + soy protein + polyethylene oxide (electrospun nanofibers) | Allyl isothiocyanate | Antimicrobial | [107] |
| Food packaging | CD-loaded polymer | β-CD + polylactic acid (electrospun nanofibers) | trans-Cinnamaldehyde | Antimicrobial | [108] |
| Food packaging | CD-loaded polymer | β-CD + zein (electrospun nanofibers) | Eucalyptus essential oil | Antimicrobial | [109] |
| Food packaging | CD-loaded polymer | β-CD + polyvinyl alcohol + lysozyme (electrospun nanofibers) | Cinnamon essential oil | Antimicrobial | [110] |
| Food packaging | CD-loaded polymer | β-CD + chitosan + polyvinyl alcohol + lysozyme (electrospun nanofibers) | Cinnamon essential oil | Antimicrobial | [111] |
| Food packaging | CD-loaded polymer | γ-CD + zein (electrospun nanofibers) | Quercetin | Antioxidant | [112] |
| Food packaging | CD-loaded polymer | HPβ-CD + gliadin (electrospun nanofibers) | Ferulic acid | Antioxidant | [113] |
| Food packaging | CD-loaded polymer | γ-CD + polyvinyl alcohol (electrospun nanofibers) | Geraniol | Antimicrobial, antioxidant and fragrant | [114] |
| Food packaging | CD-loaded polymer | β-CD + pullulan (electrospun nanofibers) | D-Limonene | Antimicrobial and fragrant | [115] |
| Food packaging | CD-loaded polymer | β-CD + chitosan | 2-Phenyl ethanol | Antimicrobial and fragrant | [116] |
| Detection of BPA | Crosslinked CD polymer | β-CD polymer film | Bisphenol A (BPA) | Enhancement of fluorescence intensity | [117] |
| Detection of NPN | (Molecularly imprinted) crosslinked CD polymer | β-CD polymer particles | N-Phenyl-1-naphtylamine | Enhancement of fluorescence intensity | [118] |
| Detection of AdCA | CD-suspended polymer | Poly(phenylene-ethylene) β-CD appended | 1-Adamantanecarboxylic acid | Shift of fluorescence peak | [119] |
| Detection of 1-phenylethylamine | CD-suspended polymer | (Stereoregular) polyphenylacetylene β-CD appended | 1-Phenylethylamine | Shift of fluorescence peak | [120] |
| Detection of chlorinated phenols | Linear CD polymer | Azo-dye modified CD-polyoxyethylene | Chlorinated phenols | Quenching of fluorescence intensity | [121] |
| Detection of nitroarenes | Crosslinked CD polymer | CD-functionalized polyarene | Trinitrophenol, nitrobenzene | Quenching of fluorescence intensity | [122] |
| Detection of captopril | Crosslinked CD polymer | β-CD functionalized poly-TFT | Captopril | Activation of fluorescence | [123] |
| Detection of benzene | Crosslinked CD polymer | CD/maleic acid copolymer | Benzene | Quartz crystal microbalance sensing | [124] |
3.2. Cyclodextrin-Based Materials for Contaminant or Toxin Removal
3.2.1. Water
3.2.2. Body and Food Matrixes
3.3. Role in the Extraction of Food-Related Compounds
3.4. Effect on Flavor and Fragrances
3.5. Cyclodextrin-Based Materials and Antimicrobials—Uses in Food Packaging
3.6. Cyclodextrin-Based Materials as Sensors for Food Safety and Health
3.6.1. Optical Sensors
3.6.2. Other CD-Based Sensors
4. Critical Overview of the Capacity, Toxicity and Current Status of CD-Based Materials
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szente, L.; Szejtli, J. Cyclodextrins as Food Ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of Cyclodextrins in Food Science. A Review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Lucia Appleton, S.; Navarro-Orcajada, S.; Martínez-Navarro, F.J.; Caldera, F.; López-Nicolás, J.M.; Trotta, F.; Matencio, A. Cyclodextrins as Anti-Inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules 2021, 11, 1384. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Effect of Hydroxypropyl-β-Cyclodextrin on the Aggregation of (E)-Resveratrol in Different Protonation States of the Guest Molecule. Food Chem. 2010, 118, 648–655. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Rubin Pedrazzo, A.; Khazaei Monfared, Y.; Dhakar, N.K.; Trotta, F. A Physicochemical, Thermodynamical, Structural and Computational Evaluation of Kynurenic Acid/Cyclodextrin Complexes. Food Chem. 2021, 356, 129639. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Matencio, A.; Vicente-Herrero, C.; García-Carmona, F.; López-Nicolás, J.M. Study of the Fluorescence and Interaction between Cyclodextrins and Neochlorogenic Acid, in Comparison with Chlorogenic Acid. Sci. Rep. 2021, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.J.; Hedges, A.R. Properties and Applications of Cyclodextrins. J. Macromol. Sci. Part A 1996, 33, 673–683. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.-S.; Khan, H.; et al. Resveratrol and Cyclodextrins, an Easy Alliance: Applications in Nanomedicine, Green Chemistry and Biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; López-Nicolás, J.M.; Núñez-Delicado, E.; Nortes-Ruipérez, D.; Sánchez-Ferrer, A.; Garciá-Carmona, F. Cyclodextrins as Hosts for Poorly Water-Soluble Compounds in Enzyme Catalysis. Appl. Biochem. Biotechnol. 1996, 61, 189–198. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Cecone, C.; López-Nicolás, J.M.; Trotta, F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Mark Bricka, R.; Dean Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Knill, C.J. Starch and Starch Containing Origins—Structure, Properties and New Technologies. Carbohydr. Polym. 2003, 54, 392. [Google Scholar] [CrossRef]
- De Souza Neves Ellendersen, L.; Milinsk, M.C.; Feroldi, M.; Volkweis Zadinelo, I.; Dena Dos Santos, L.; Bolzón De Muniz, G.I.; Gasparrini, L.J.; Alves, H.J. Biopolymer Foam for Remediation of Aquatic Environments Contaminated with Particulates and Heavy Metals. J. Environ. Chem. Eng. 2018, 6, 6131–6138. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of Wastewater Treatment Methods with Special Focus on Biopolymer Chitin-Chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Vebrel, J. Removal of Organic Pollutants from Aqueous Solutions by Adsorbents Prepared from an Agroalimentary By-Product. Bioresour. Technol. 2006, 97, 2173–2181. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. The Classification and Application of Cyclodextrin Polymers: A Review. New J. Chem. 2020, 44, 9137–9148. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The Application and Prospects of Cyclodextrin Inclusion Complexes and Polymers in the Food Industry: A Review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Traisnel, M. Remediation of Heavy Metals by Biomolecules: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1644–1704. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-Based Polymer Materials: From Controlled Synthesis to Applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Matencio, A.; Guerrero-Rubio, M.A.; Gandía-Herrero, F.; García-Carmona, F.; López-Nicolás, J.M. Nanoparticles of Betalamic Acid Derivatives with Cyclodextrins. Physicochemistry, Production Characterization and Stability. Food Hydrocoll. 2020, 110, 106176. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Kulkarni, A.; Defrees, K.; Hyun, S.H.; Thompson, D.H. Pendant Polymer:Amino-β-Cyclodextrin:SiRNA Guest:Host Nanoparticles as Efficient Vectors for Gene Silencing. J. Am. Chem. Soc. 2012, 134, 7596–7599. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Feng, A.; Yuan, J. Polymeric Nanocarriers Based on Cyclodextrins for Drug Delivery: Host-Guest Interaction as Stimuli Responsive Linker. Mol. Pharm. 2017, 14, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J.; Hetzer, M.; Ritter, H.; Barner-Kowollik, C. Complex Macromolecular Architecture Design via Cyclodextrin Host/Guest Complexes. Prog. Polym. Sci. 2014, 39, 235–249. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef]
- Fischer, F.; Wenzel, K.J.; Rademann, K.; Emmerling, F. Quantitative Determination of Activation Energies in Mechanochemical Reactions. Phys. Chem. Chem. Phys. 2016, 18, 23320–23325. [Google Scholar] [CrossRef] [Green Version]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-Based Supramolecular Systems for Drug Delivery: Recent Progress and Future Perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogoshi, T.; Harada, A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors 2008, 8, 4961–4982. [Google Scholar] [CrossRef] [Green Version]
- Van De Manakker, F.; Vermonden, T.; Van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z. Cyclodextrin Polymers: Structure, Synthesis, and Use as Drug Carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Seidi, F.; Jin, Y.; Xiao, H. Polycyclodextrins: Synthesis, Functionalization, and Applications. Carbohydr. Polym. 2020, 242. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Ekberg, B.; Sellergren, B.; Olsson, L.; Mosbach, K. The Synthesis of Water-Soluble Polymers of β-cyclodextrin and Their Use in Aqueous Two-Phase Systems. Carbohydr. Polym. 1989, 10, 183–188. [Google Scholar] [CrossRef]
- Rubin Pedrazzo, A.; Smarra, A.; Caldera, F.; Musso, G.; Dhakar, N.K.; Cecone, C.; Hamedi, A.; Corsi, I.; Trotta, F. Eco-Friendly β-Cyclodextrin and Linecaps Polymers for the Removal of Heavy Metals. Polymers 2019, 11, 1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Huang, W.-Q.; Hu, J.-L. Water-Insoluble β-Cyclodextrin Polymer Crosslinked by Citric Acid: Synthesis and Adsorption Properties toward Phenol and Methylene Blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201. [Google Scholar] [CrossRef]
- Trotta, F.; Shende, P.; Biasizzo, M. Method for Preparing Dextrin Nanosponges. WO2012147069A1, 28 April 2011. [Google Scholar]
- Trotta, F. Cyclodextrin Nanosponges and Their Applications. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applicatio; Wiley: Hoboken, NJ, USA, 2011; pp. 323–342. [Google Scholar] [CrossRef]
- Biopolymkres, L.D.P.; Paris, C. Polycondensation of cyclodextrins with epichlorohydrin influence of reaction conditions on the polymer structure. Macromol. Symposia. Basel Hüthig Wepf Verl. 1997, 122, 229–234. [Google Scholar]
- Fülöp, Z.; Kurkov, S.V.; Nielsen, T.T.; Larsen, K.L.; Loftsson, T. Self-Assembly of Cyclodextrins: Formation of Cyclodextrin Polymer Based Nanoparticles. J. Drug Deliv. Sci. Technol. 2012, 22, 215–221. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-Based Nanosponges: A Critical Review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Caldera, F.; Cavalli, R.; Mele, A.; Punta, C.; Melone, L.; Castiglione, F.; Rossi, B.; Ferro, M.; Crupi, V.; et al. Synthesis and Characterization of a Hyper-Branched Water-Soluble β-Cyclodextrin Polymer. Beilstein J. Org. Chem. 2014, 10, 2586–2593. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hwang, S.J.; Davis, M.E. New Class of Polymers for the Delivery of Macromolecular Therapeutics. Bioconjug. Chem. 1999, 10, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Singh, P.; Singhal, A. Alka Cyclodextrin-Based Nanostructured Materials for Sustainable Water Remediation Applications. Environ. Sci. Pollut. Res. 2020, 27, 32432–32448. [Google Scholar] [CrossRef]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A Review on the Recent Development of Cyclodextrin-Based Materials Used in Oilfield Applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, M. Synthesis of Substituted Cyclodextrins. Environ. Chem. Lett. 2019, 17, 49–63. [Google Scholar] [CrossRef]
- Popielarski, S.R.; Mishra, S.; Davis, M.E. Structural Effects of Carbohydrate-Containing Polycations on Gene Delivery 3 Cyclodextrin Type and Functionalization. Bioconjug. Chem. 2003, 14, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reineke, T.M.; Davis, M.E. Structural Effects of Carbohydrate-Containing Polycations on Gene Delivery 1 Carbohydrate Size and Its Distance from Charge Centers. Bioconjug. Chem. 2003, 14, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Guo, X.; Abdala, A.A.; May, B.L.; Lincoln, S.F.; Khan, S.A.; Prud, R.K. Novel Associative Polymer Networks Based on Cyclodextrin Inclusion Compounds. Macromolecules 2005, 38, 3037–3040. [Google Scholar] [CrossRef]
- Ramírez, H.L.; Valdivia, A.; Cao, R.; Torres-Labandeira, J.J.; Fragoso, A.; Villalonga, R. Cyclodextrin-Grafted Polysaccharides as Supramolecular Carrier Systems for Naproxen. Bioorgan. Med. Chem. Lett. 2006, 16, 1499–1501. [Google Scholar] [CrossRef]
- Yi, Z.; Yu, L. Supramolecular Assemblies of Multi-Charged Cyclodextrins. Chin. J. Org. Chem. 2020, 40, 3802. [Google Scholar] [CrossRef]
- Yao, X.; Mu, J.; Zeng, L.; Lin, J.; Nie, Z.; Jiang, X.; Huang, P. Stimuli-Responsive Cyclodextrin-Based Nanoplatforms for Cancer Treatment and Theranostics. Mater. Horiz. 2019, 6, 846–870. [Google Scholar] [CrossRef]
- Hu, X.; Shang, M.; Wang, J.; Liu, L.; Lu, W.; Ye, L.; Wang, J. Mass Formation of α-Cyclodextrin Hexagonal Rods by the Direct Solvent Evaporation Strategy. ACS Appl. Bio Mater. 2021, 4, 8033–8038. [Google Scholar] [CrossRef]
- Mayumi, K.; Ito, K. Structure and Dynamics of Polyrotaxane and Slide-Ring Materials. Polymer 2010, 51, 959–967. [Google Scholar] [CrossRef] [Green Version]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and Antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, É. Cyclodextrin-Enabled Polymer Composites for Packaging. Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [Green Version]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of Solubility Enhancement, Antioxidant Activity, and Cytotoxicity Studies of Kynurenic Acid Loaded Cyclodextrin Nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [Green Version]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of Oxyresveratrol Complexes with Insoluble Cyclodextrin Based Nanosponges: Developing a Novel Way to Obtain Their Complexation Constants and Application in an Anticancer Study. Carbohydr. Polym. 2020, 231, 115763. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, Á.; Garcia-Fandino, R. The Lord of the NanoRings: Cyclodextrins and the Battle against SARS-CoV-2. Int. J. Pharm. 2020, 588, 119689. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; González-Ramón, A.; García-Carmona, F.; López-Nicolás, J.M. Recent Advances in the Treatment of Niemann Pick Disease Type C: A Mini-Review. Int. J. Pharm. 2020, 584, 119440. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-H.; Chung, T.-W.; Giridhar, R.; Wu, W.-T. Immobilization of β-Cyclodextrin in Chitosan Beads for Separation of Cholesterol from Egg Yolk. Food Res. Int. 2004, 37, 217–223. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, H.-S.; Kwak, H.-S. Optimization of Cholesterol Removal by Crosslinked Beta-Cyclodextrin in Egg Yolk. Food Sci. Biotechnol. 2005, 14, 793–797. [Google Scholar]
- Han, E.-M.; Sangjin, K.; Ahn, J.; Kwak, H.-S. Cholesterol Removal from Homogenized Milk with Crosslinked Beta-Cyclodextrin by Adipic Acid. Asian-Australas. J. Anim. Sci. 2005, 18, 1794–1799. [Google Scholar] [CrossRef]
- Tahir, M.N.; Kwon, C.; Jeong, D.; Cho, E.; Paik, S.R.; Jung, S. Cholesterol Reduction from Milk Using β-Cyclodextrin Immobilized on Glass. J. Dairy Sci. 2013, 96, 4191–4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köse, K.; Mavlan, M.; Uzun, L.; Youngblood, J.P. Cholesterol Removal via Cyclodextrin-Decoration on Cellulose Nanocrystal (CNC)-Grafted Poly(HEMA-GMA) Nanocomposite Adsorbent. Cellulose 2021, 28, 471–487. [Google Scholar] [CrossRef]
- Jung, S.; Tahir, M.N.; Paik, S.R.; Jeong, D.; Kwon, C. Beta-Cyclodextrin Immobilized on Glass and Cholesterol Removal Using the Same. U.S. Patent 9,049,871, 9 June 2015. [Google Scholar]
- Kwak, H.-S.; Kim, S.-H.; Jeong, T.-H.; Han, E.-m. Method for Crosslinking of β-Cyclodextrin for Cholesterol Removal and Regeneration of the Same. KR100791978B1, 4 January 2008. [Google Scholar]
- Miao, J.; Guo, B.; Liu, Z.; Yu, P.; Ren, L.; Xiao, Y.; Cai, T.; Wang, H. Low Cholesterol Dairy Product and Crosslinked Beta-Cyclodextrin, Preparation Method and Application Thereof. CN103601901A, 26 February 2014. [Google Scholar]
- Alonso, L.; Calvo, M.V.; Fontecha, J. A Scale-up Process for the Manufacture of Reduced-Cholesterol Butter Using Beta-Cyclodextrin. J. Food Process Eng. 2019, 42, e13009. [Google Scholar] [CrossRef]
- Alonso, L.; Calvo, M.V.; Fontecha, J. The Influence of β-Cyclodextrin on the Reduction of Cholesterol Content in Egg and Duck Liver Pâté. Foods 2019, 8, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Li, Y.; Li, Q.; Du, Q.; Wang, D.; Sui, K.; Wang, C.; Li, H.; Xia, Y. Kinetic, Isotherm and Thermodynamic Studies for Removal of Methylene Blue Using β-Cyclodextrin/Activated Carbon Aerogels. J. Polym. Environ. 2018, 26, 3362–3370. [Google Scholar] [CrossRef]
- Gomez-Maldonado, D.; Reynolds, A.M.; Johansson, L.-S.; Burnett, D.J.; Ramapuram, J.B.; Waters, M.N.; Vega Erramuspe, I.B.; Peresin, M.S. Fabrication of Aerogels from Cellulose Nanofibril Grafted with β-Cyclodextrin for Capture of Water Pollutants. J. Porous. Mater. 2021, 28, 1725–1736. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J. Removal of Organic Contaminants from Water Using Nanosponge Cyclodextrin Polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin Nanosponges as Adsorbent Material to Remove Hazardous Pollutants from Water: The Case of Ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Yang, A.; Ching, C.; Easler, M.; Helbling, D.E.; Dichtel, W.R. Cyclodextrin Polymers with Nitrogen-Containing Tripodal Crosslinkers for Efficient PFAS Adsorption. ACS Mater. Lett. 2020, 2, 1240–1245. [Google Scholar] [CrossRef]
- Choi, S.H.; Chung, J.W.; Priestley, R.D.; Kwak, S.-Y. Functionalization of Polysulfone Hollow Fiber Membranes with Amphiphilic β-Cyclodextrin and Their Applications for the Removal of Endocrine Disrupting Plasticizer. J. Membr. Sci. 2012, 409–410, 75–81. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Wang, C.; Zhang, K.; Lin, D.; Kong, L.; Liu, J. Rapid Adsorption of Pb, Cu and Cd from Aqueous Solutions by β-Cyclodextrin Polymers. Appl. Surf. Sci. 2017, 426, 29–39. [Google Scholar] [CrossRef]
- Poór, M.; Faisal, Z.; Zand, A.; Bencsik, T.; Lemli, B.; Kunsági-Máté, S.; Szente, L. Removal of Zearalenone and Zearalenols from Aqueous Solutions Using Insoluble Beta-Cyclodextrin Bead Polymer. Toxins 2018, 10, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fliszár-Nyúl, E.; Szabó, Á.; Szente, L.; Poór, M. Extraction of Mycotoxin Alternariol from Red Wine and from Tomato Juice with Beta-Cyclodextrin Bead Polymer. J. Mol. Liq. 2020, 319, 114180. [Google Scholar] [CrossRef]
- Varan, C.; Anceschi, A.; Sevli, S.; Bruni, N.; Giraudo, L.; Bilgiç, E.; Korkusuz, P.; İskit, A.B.; Trotta, F.; Bilensoy, E. Preparation and Characterization of Cyclodextrin Nanosponges for Organic Toxic Molecule Removal. Int. J. Pharm. 2020, 585, 119485. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.E.; Buslig, B.S. Selective Removal of Bitter Compounds from Grapefruit Juice and from Aqueous Solution with Cyclodextrin Polymers and with Amberlite XAD-4. J. Agric. Food Chem. 1986, 34, 837–840. [Google Scholar] [CrossRef]
- Gong, A.; Ping, W.; Wang, J.; Zhu, X. Cyclodextrin Polymer/Fe3O4 Nanocomposites as Solid Phase Extraction Material Coupled with UV–Vis Spectrometry for the Analysis of Rutin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis Cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Sui, S.; Ference, C.; Zhang, Y.; Sun, S.; Zhou, N.; Zhu, W.; Zhou, K. Antimicrobial and Mechanical Properties of β-Cyclodextrin Inclusion with Essential Oils in Chitosan Films. J. Agric. Food Chem. 2014, 62, 8914–8918. [Google Scholar] [CrossRef]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a New Antibacterial Bio-Nano-Material for Food-Packaging by Synergistic Action of Cyclodextrin and Microfibrillated Cellulose. Innov. Food Sci. Emerg. Technol. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- Buendía−Moreno, L.; Sánchez−Martínez, M.J.; Antolinos, V.; Ros−Chumillas, M.; Navarro−Segura, L.; Soto−Jover, S.; Martínez−Hernández, G.B.; López−Gómez, A. Active Cardboard Box with a Coating Including Essential Oils Entrapped within Cyclodextrins and/or Halloysite Nanotubes. A Case Study for Fresh Tomato Storage. Food Control 2020, 107, 106763. [Google Scholar] [CrossRef]
- de Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled Release of Carvacrol and Curcumin: Bio-Based Food Packaging by Synergism Action of TEMPO-Oxidized Cellulose Nanocrystals and Cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Goni, L.; González-Gaitano, G.; Vélaz, I. Cyclodextrin-Grafted Nanoparticles as Food Preservative Carriers. Int. J. Pharm. 2020, 588, 119664. [Google Scholar] [CrossRef]
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of Coriander Essential Oil in Cyclodextrin Nanosponges: A New Strategy to Promote Its Use in Controlled-Release Active Packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
- Simionato, I.; Domingues, F.C.; Nerín, C.; Silva, F. Encapsulation of Cinnamon Oil in Cyclodextrin Nanosponges and Their Potential Use for Antimicrobial Food Packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef] [PubMed]
- Brasil, I.M.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.E.; Moreira, R.G. Polysaccharide-Based Multilayered Antimicrobial Edible Coating Enhances Quality of Fresh-Cut Papaya. LWT-Food Sci. Technol. 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Mantilla, N.; Castell-Perez, M.E.; Gomes, C.; Moreira, R.G. Multilayered Antimicrobial Edible Coating and Its Effect on Quality and Shelf-Life of Fresh-Cut Pineapple (Ananas Comosus). LWT-Food Sci. Technol. 2013, 51, 37–43. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Bazzano, M.; Sangermano, M.; Pisano, R. Inclusion Complexes Dispersed in Polystyrene-Based Labels for Fruit Ripening on Demand. Int. J. Food Sci. Technol. 2018, 53, 389–394. [Google Scholar] [CrossRef]
- Neoh, T.L.; Ariyanto, H.D.; Galvan, P.M.; Yoshii, H. Controlled Release of 1-Methylcyclopropene from Its Functionalised Electrospun Fibres under Constant and Linearly Ramped Humidity. Food Addit. Contam. Part A 2017, 34, 1690–1702. [Google Scholar] [CrossRef]
- Lang, T. Investigation of the Encapsulation Efficiency of Hexanal in Y-Cyclodextrin Metal Organic Frameworks. Mater. Eng. 2019. [Google Scholar]
- Kwak, S.-Y.; Jung, S.-J.; Chung, J.-W. Poly(Vinyl Chloride) Product Containing Cyclodextrin Derivatives with Suppression of the Migration of Plasticizer and Manufacturing Method Thereof. U.S. Patent 8,008,375, 30 August 2011. [Google Scholar]
- Jia, X.; Yang, N.; Qi, X.; Chen, L.; Zhao, Y. Adsorptive Removal of Cholesterol by Biodegradable Zein-Graft-β-Cyclodextrin Film. Int. J. Biol. Macromol. 2020, 155, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, E.J.; Ahn, D.U. Electrospinning of Tri-Acetyl-β-Cyclodextrin (TA-β-CD) Functionalized Low-Density Polyethylene to Minimize Sulfur Odor Volatile Compounds. Food Packag. Shelf Life 2018, 18, 107–114. [Google Scholar] [CrossRef]
- Vega-Lugo, A.-C.; Lim, L.-T. Controlled Release of Allyl Isothiocyanate Using Soy Protein and Poly(Lactic Acid) Electrospun Fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/β-Cyclodextrin Fibers as an Antimicrobial Wound Dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini, Â.M.; Pinto, V.Z.; Lim, L.-T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial Electrospun Ultrafine Fibers from Zein Containing Eucalyptus Essential Oil/Cyclodextrin Inclusion Complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef]
- Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W.Y.; Zong, M.H.; Wu, H. Enhancement of the Antimicrobial Activity of Cinnamon Essential Oil-Loaded Electrospun Nanofilm by the Incorporation of Lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and Antifungal Properties of β-Cyclodextrin Microcapsules and Nanofibre Films Containing Cinnamon and Oregano Essential Oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Uyar, T. Antioxidant Electrospun Zein Nanofibrous Web Encapsulating Quercetin/Cyclodextrin Inclusion Complex. J. Mater. Sci. 2018, 53, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Sharif, N.; Golmakani, M.-T.; Niakousari, M.; Hosseini, S.M.H.; Ghorani, B.; Lopez-Rubio, A. Active Food Packaging Coatings Based on Hybrid Electrospun Gliadin Nanofibers Containing Ferulic Acid/Hydroxypropyl-Beta-Cyclodextrin Inclusion Complexes. Nanomaterials 2018, 8, 919. [Google Scholar] [CrossRef] [Green Version]
- Kayaci, F.; Sen, H.S.; Durgun, E.; Uyar, T. Functional Electrospun Polymeric Nanofibers Incorporating Geraniol–Cyclodextrin Inclusion Complexes: High Thermal Stability and Enhanced Durability of Geraniol. Food Res. Int. 2014, 62, 424–431. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Mascheroni, E.; Cosio, M.S.; Piergiovanni, L.; Benedetti, S.; Ortenzi, M.; Schiraldi, A.; Mannino, S. Encapsulation of R(+)Limonene in Edible Electrospun Nanofibers. Chem. Eng. Trans. 2013, 32, 1771–1776. [Google Scholar] [CrossRef]
- Zarandona, I.; Barba, C.; Guerrero, P.; de la Caba, K.; Maté, J. Development of Chitosan Films Containing β-Cyclodextrin Inclusion Complex for Controlled Release of Bioactives. Food Hydrocoll. 2020, 104, 105720. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, H.; Wei, Y.; Lin, J.-M. A Reversible Fluorescence Sensor Based on Insoluble β-Cyclodextrin Polymer for Direct Determination of Bisphenol A (BPA). Sens. Actuators B Chem. 2006, 114, 565–572. [Google Scholar] [CrossRef]
- Ng, S.M.; Narayanaswamy, R. Molecularly Imprinted β-Cyclodextrin Polymer as Potential Optical Receptor for the Detection of Organic Compound. Sens. Actuators B Chem. 2009, 139, 156–165. [Google Scholar] [CrossRef]
- Ogoshi, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Cyclodextrin-Grafted Poly (Phenylene Ethynylene) with Chemically-Responsive Properties. Chem. Commun. 2006, 35, 3702–3704. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Sato, O. Switching of a Macromolecular Helicity for Visual Distinction of Molecular Recognition Events. J. Am. Chem. Soc. 2001, 123, 8159–8160. [Google Scholar] [CrossRef] [PubMed]
- Ncube, P.; Krause, R.W.; Mamba, B.B. Fluorescent Sensing of Chlorophenols in Water Using an Azo Dye Modified β-Cyclodextrin Polymer. Sensors 2011, 11, 4598–4608. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M.K.; Wang, S.; Wang, Q.; Wang, B.; Wilson, L.D. A Porous β-Cyclodextrin-Based Terpolymer Fluorescence Sensor for in Situ Trinitrophenol Detection. RSC Adv. 2019, 9, 8073–8080. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Peng, J.; Meng, X.; Huang, T.; Zhang, J.; He, H. Turn-on Fluorescent Detection of Captopril in Urine Samples Based on Hydrophilic Hydroxypropyl β-Cyclodextrin Polymer. Anal. Bioanal. Chem. 2018, 410, 7373–7384. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-F.; Syu, M.-J.; Teng, H.-S.; Chou, S.-K.; Chang, Y.-S. Preparation and Identification of β-Cyclodextrin Polymer Thin Film for Quartz Crystal Microbalance Sensing of Benzene, Toluene, and p-Xylene. Sens. Actuators B Chem. 2008, 132, 319–326. [Google Scholar] [CrossRef]
- Xing, W.; Ngo, H.H.; Kim, S.H.; Guo, W.S.; Hagare, P. Adsorption and Bioadsorption of Granular Activated Carbon (GAC) for Dissolved Organic Carbon (DOC) Removal in Wastewater. Bioresour. Technol. 2008, 99, 8674–8678. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X.; Zhang, X. Cyclic Molecule Aerogels: A Robust Cyclodextrin Monolith with Hierarchically Porous Structures for Removal of Micropollutants from Water. J. Mater. Chem. A 2017, 5, 4308–4313. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Pillay, K.; Yangkou Mbianda, X. Nanosponge Cyclodextrin Polyurethanes and Their Modification with Nanomaterials for the Removal of Pollutants from Waste Water: A Review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Hoti, G.; Appleton, S.L.; Pedrazzo, A.R.; Cecone, C.; Matencio, A.; Trotta, F.; Caldera, F. Strategies to Develop Cyclodextrin-Based Nanosponges for Smart Drug Delivery; IntechOpen: London, UK, 2021; ISBN 978-1-83969-539-1. [Google Scholar]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-Cyclodextrin as a Green Co-Solvent in the Aqueous Extraction of Polyphenols from Waste Orange Peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-Assisted Extraction of Phenolic Compounds: Current Research and Future Prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential Oil–Cyclodextrin Complexes: An Updated Review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Celebioglu, A.; Sen, H.S.; Durgun, E.; Uyar, T. Molecular Entrapment of Volatile Organic Compounds (VOCs) by Electrospun Cyclodextrin Nanofibers. Chemosphere 2016, 144, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Del-Toro-Sánchez, L.; Alvarez-Parrilla, E.; González-Aguilar, G.A. High Relative Humidity In-Package of Fresh-Cut Fruits and Vegetables: Advantage or Disadvantage Considering Microbiological Problems and Antimicrobial Delivering Systems? J. Food Sci. 2008, 73, R41–R47. [Google Scholar] [CrossRef] [PubMed]
- Noruzi, M. Electrospun Nanofibres in Agriculture and the Food Industry: A Review. J. Sci. Food Agric. 2016, 96, 4663–4678. [Google Scholar] [CrossRef]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced Thermal Stability of Eugenol by Cyclodextrin Inclusion Complex Encapsulated in Electrospun Polymeric Nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Jones, M.E.; Smith, R.E. Volatile Organic Compounds in Foods: A Five Year Study. J. Agric. Food Chem. 2003, 51, 8120–8127. [Google Scholar] [CrossRef] [PubMed]
- Heikes, D.L.; Jensen, S.R.; Fleming-Jones, M.E. Purge and Trap Extraction with GC-MS Determination of Volatile Organic Compounds in Table-Ready Foods. J. Agric. Food Chem. 1995, 43, 2869–2875. [Google Scholar] [CrossRef]
- Piringer, O.G.; Baner, A.L. Permeation of Gases, Water Vapor and Volatile Organic Compounds. Plast. Packag. Mater. Food Barrier Funct. Mass Transp. Qual. Assur. Legis. 2000. [Google Scholar]
- Hwang, J.B.; Lee, S.; Yeum, J.; Kim, M.; Choi, J.C.; Park, S.-J.; Kim, J. HS-GC/MS Method Development and Exposure Assessment of Volatile Organic Compounds from Food Packaging into Food Simulants. Food Addit. Contam. Part A 2019, 36, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Sanagi, M.M.; Ling, S.L.; Nasir, Z.; Ibrahim, W.A.W.; Naim, A.A. Determination of Residual Volatile Organic Compounds Migrated from Polystyrene Food Packaging into Food Simulant by Headspace Solid Phase Microextraction—Gas Chromatography. Malays. J. Anal. Sci. 2008, 12, 542–551. [Google Scholar]
- Hodgson, S.C.; Casey, R.J.; Bigger, S.W.; Scheirs, J. Review of Volatile Organic Compounds Derived from Polyethylene. Polym.-Plast. Technol. Eng. 2000, 39, 845–874. [Google Scholar] [CrossRef]
- Sadler, G.; Pierce, D.; Lawson, A.; Suvannunt, D.; Senthil, V. Evaluating Organic Compound Migration in Poly (Ethylene Terephthalate): A Simple Test with Implications for Polymer Recycling. Food Addit. Contam. 1996, 13, 979–989. [Google Scholar] [CrossRef]
- Artabe, A.E.; Cunha-Silva, H.; Barranco, A. Enzymatic Assays for the Assessment of Toxic Effects of Halogenated Organic Contaminants in Water and Food. A Review. Food Chem. Toxicol. 2020, 145, 111677. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, H.; Ohnishi, Y.; Rosenkranz, H.S. Mutagenicity and Carcinogenicity of Nitroarenes and Their Sources in the Environment. CRC Crit. Rev. Toxicol. 1986, 17, 23–58. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Sharma, B.; Negi, V.; Aswal, R.; Singh, P.; Singh, R.; Dobhal, R. Pharmaceutical Compounds in Drinking Water. J. Xenobiotics 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xue, Z.; Zhao, X.; Chen, W.; Mu, T. Green Synthesis of Porous β-Cyclodextrin Polymers for Rapid and Efficient Removal of Organic Pollutants and Heavy Metal Ions from Water. New J. Chem. 2018, 42, 16154–16161. [Google Scholar] [CrossRef]
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green Synthesis of a Magnetic β-Cyclodextrin Polymer for Rapid Removal of Organic Micro-Pollutants and Heavy Metals from Dyeing Wastewater. Environ. Res. 2020, 180, 108796. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzo, A.R.; Caldera, F.; Zanetti, M.; Appleton, S.L.; Dhakar, N.K.; Trotta, F. Mechanochemical Green Synthesis of Hyper-Crosslinked Cyclodextrin Polymers. Beilstein J. Org. Chem. 2020, 16, 1554–1563. [Google Scholar] [CrossRef]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for Selective Modifications of Cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yi, Y.; Chen, J. Recent Advances for Cyclodextrin-Based Materials in Electrochemical Sensing. TrAC Trends Anal. Chem. 2016, 80, 232–241. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Ellagic Acid—Borax Fluorescence Interaction: Application for Novel Cyclodextrin-Borax Nanosensors for Analyzing Ellagic Acid in Food Samples. Food Funct. 2018, 9, 3683–3687. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly Imprinted Polymers as Antibody and Receptor Mimics for Assays, Sensors and Drug Discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef]
- Asanuma, H.; Kakazu, M.; Shibata, M.; Hishiya, T. Molecularly Imprinted Polymer of β-Cyclodextrin for the Efficient Recognition of Cholesterol. Chem. Commun. 1997, 20, 1971–1972. [Google Scholar] [CrossRef]
- Hishiya, T.; Shibata, M.; Kakazu, M.; Asanuma, H.; Komiyama, M. Molecularly Imprinted Cyclodextrins as Selective Receptors for Steroids. Macromolecules 1999, 32, 2265–2269. [Google Scholar] [CrossRef]
- Maeda, K.; Mochizuki, H.; Watanabe, M.; Yashima, E. Switching of Macromolecular Helicity of Optically Active Poly (Phenylacetylene) s Bearing Cyclodextrin Pendants Induced by Various External Stimuli. J. Am. Chem. Soc. 2006, 128, 7639–7650. [Google Scholar] [CrossRef] [PubMed]
- McCallum, J.J. Piezoelectric Devices for Mass and Chemical Measurements: An Update. A Review. Analyst 1989, 114, 1173–1189. [Google Scholar] [CrossRef]
- He, J.-L.; Yang, Y.; Yang, X.; Liu, Y.-L.; Liu, Z.-H.; Shen, G.-L.; Yu, R.-Q. β-Cyclodextrin Incorporated Carbon Nanotube-Modified Electrode as an Electrochemical Sensor for Rutin. Sens. Actuators B Chem. 2006, 114, 94–100. [Google Scholar] [CrossRef]
- Ogoshi, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Chemically-Responsive Sol-Gel Transition of Supramolecular Single-Walled Carbon Nanotubes (SWNTs) Hydrogel Made by Hybrids of SWNTs and Cyclodextrins. J. Am. Chem. Soc. 2007, 129, 4878–4879. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Kong, L.-T.; Yang, R.; Wang, L.; Liu, J.-H.; Huang, X.-J. Electrochemical Impedance Determination of Polychlorinated Biphenyl Using a Pyrenecyclodextrin-Decorated Single-Walled Carbon Nanotube Hybrid. Chem. Commun. 2011, 47, 5340–5342. [Google Scholar] [CrossRef]
- Yang, L.; Fan, S.; Deng, G.; Li, Y.; Ran, X.; Zhao, H.; Li, C.-P. Bridged β-Cyclodextrin-Functionalized MWCNT with Higher Supramolecular Recognition Capability: The Simultaneous Electrochemical Determination of Three Phenols. Biosens. Bioelectron. 2015, 68, 617–625. [Google Scholar] [CrossRef]
- Agnihotri, N.; Chowdhury, A.D.; De, A. Non-Enzymatic Electrochemical Detection of Cholesterol Using β-Cyclodextrin Functionalized Graphene. Biosens. Bioelectron. 2015, 63, 212–217. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host-Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ong, W.; Román, E.; Lynn, M.J.; Kaifer, A.E. Cyclodextrin-Modified Gold Nanospheres. Langmuir 2000, 16, 3000–3002. [Google Scholar] [CrossRef]
- Kikuchi, T.; Narita, M.; Hamada, F. Synthesis of Bis Dansyl-Modified β-Cyclodextrin Liner Trimer Having Multi-Recognition Sites and High Hydrophobic Environment. Tetrahedron 2001, 57, 9317–9324. [Google Scholar] [CrossRef]
- Sasaki, K.; Nagasaka, M.; Kuroda, Y. New Cyclodextrin Dimer and Trimer: Formation of Biphenyl Excimer and Their Molecular Recognition. Chem. Commun. 2001, 24, 2630–2631. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Matencio, A.; Guerrero-Rubio, M.A.; Caldera, F.; Cecone, C.; Trotta, F.; García-Carmona, F.; López-Nicolás, J.M. Lifespan Extension in Caenorhabditis Elegans by Oxyresveratrol Supplementation in Hyper-Branched Cyclodextrin-Based Nanosponges. Int. J. Pharm. 2020, 589, 119862. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the Cytotoxicity of β-Cyclodextrin Derivatives: Evidence for the Role of Cholesterol Extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matencio, A.; Rubin Pedrazzo, A.; Difalco, A.; Navarro-Orcajada, S.; Khazeai Monfared, Y.; Conesa, I.; Rezayat, A.; López-Nicolás, J.M.; Trotta, F. Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers 2021, 13, 4226. https://doi.org/10.3390/polym13234226
Matencio A, Rubin Pedrazzo A, Difalco A, Navarro-Orcajada S, Khazeai Monfared Y, Conesa I, Rezayat A, López-Nicolás JM, Trotta F. Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers. 2021; 13(23):4226. https://doi.org/10.3390/polym13234226
Chicago/Turabian StyleMatencio, Adrián, Alberto Rubin Pedrazzo, Alessandro Difalco, Silvia Navarro-Orcajada, Yousef Khazeai Monfared, Irene Conesa, Azam Rezayat, José Manuel López-Nicolás, and Francesco Trotta. 2021. "Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues" Polymers 13, no. 23: 4226. https://doi.org/10.3390/polym13234226
APA StyleMatencio, A., Rubin Pedrazzo, A., Difalco, A., Navarro-Orcajada, S., Khazeai Monfared, Y., Conesa, I., Rezayat, A., López-Nicolás, J. M., & Trotta, F. (2021). Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers, 13(23), 4226. https://doi.org/10.3390/polym13234226