Formulation and Evaluation of Chitosan/NaCl/Maltodextrin Microparticles as a Saltiness Enhancer: Study on the Optimization of Excipients for the Spray-Drying Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Degree of Deacetylation (DD) and Molecular Weight of Chitosan
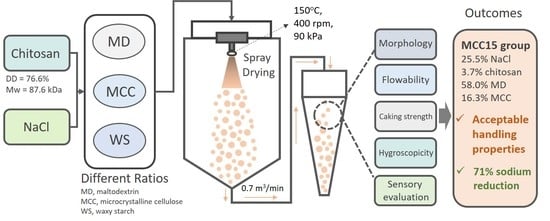
2.4. Preparation of Chitosan/NaCl/Maltodextrin Microparticles by Spray Drying
2.5. X-ray Diffraction
2.6. Scanning Electron Microscopy (SEM)
2.7. Moisture, Sodium Chloride, and Acetic Acid Content of Microparticles
2.8. Densities and Flowabilities of Microparticles
2.9. Hygroscopicity Assessment
2.10. Determination of Caking Strength
2.11. Dissolution Rate in Artificial Saliva
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Chitosan and Its Microparticles
3.1.1. The Yields of Chitosan/NaCl/Maltodextrin Microparticles
3.1.2. Moisture, NaCl, and Acetic Acid Content of Chitosan/NaCl/Maltodextrin Microparticles
3.1.3. Morphology and Particle Size
3.1.4. X-ray Diffraction
3.2. Densities and Flowability of Chitosan/NaCl/Maltodextrin Microparticles
3.3. Hygroscopicity and Caking Strength
3.4. Dissolution Rates of NaCl in the Microparticles
3.5. Sensory Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mente, A.; O’Donnell, M.; Yusuf, S. Sodium intake and health: What should we recommend based on the current evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef] [PubMed]
- Kare, M. Biological and Behavioral Aspects of Salt Intake, 1st ed.; Elsevier: Cambridge, MA, USA, 2012. [Google Scholar]
- Capewell, S.; Kypridemos, C. Socioeconomic inequalities in dietary sodium intake: Upstream versus downstream interventions. Am. J. Public Health 2017, 107, 499–500. [Google Scholar] [CrossRef]
- World Health Organization. Salt Reduction. Available online: https://www.who.int/en/news-room/factsheets/detail/salt-reduction (accessed on 9 May 2020).
- Cobiac, L.J.; Vos, T.; Veerman, J.L. Cost-effectiveness of interventions to reduce dietary salt intake. Heart 2010, 96, 1920–1925. [Google Scholar] [CrossRef]
- Martikainen, J.A.; Soini, E.J.; Laaksonen, D.E.; Niskanen, L. Health economic consequences of reducing salt intake and replacing saturated fat with polyunsaturated fat in the adult Finnish population: Estimates based on the FINRISK and FINDIET studies. Eur. J. Clin. Nutr. 2011, 65, 1148–1155. [Google Scholar] [CrossRef] [Green Version]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppu, U.; Hopia, A.; Pohjanheimo, T.; Rotola-Pukkila, M.; Mäkinen, S.; Pihlanto, A.; Sandell, M. Effect of salt reduction on consumer acceptance and sensory quality of food. Foods 2017, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Desmond, E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006, 74, 188–196. [Google Scholar] [CrossRef]
- Sinopoli, D.A.; Lawless, H.T. Taste properties of potassium chloride alone and in mixtures with sodium chloride using a check-all-that-apply method. J. Food Sci. 2012, 77, S319–S322. [Google Scholar] [CrossRef] [PubMed]
- Kilcast, D.; Den Ridder, C. Sensory issues in reducing salt in food products. In Reducing Salt in Foods; Kilcast, D., Angus, F., Eds.; Woodhead Publishing: Sawston, UK, 2007; Volume 10, pp. 201–220. [Google Scholar]
- Koliandris, A.L.; Morris, C.; Hewson, L.; Hort, J.; Taylor, A.J.; Wolf, B. Correlation between saltiness perception and shear flow behaviour for viscous solutions. Food Hydrocol. 2010, 24, 792–799. [Google Scholar] [CrossRef]
- Moncada, M.; Astete, C.; Sabliov, C.; Olson, D.; Boeneke, C.; Aryana, K.J. Nano spray-dried sodium chloride and its effects on the microbiological and sensory characteristics of surface-salted cheese crackers. J. Dairy Sci. 2015, 98, 5946–5954. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.Y.; Kim, B.; Chun, J.Y.; Choi, M.J. Effect of spray-drying process on physical properties of sodium chloride/maltodextrin complexes. Powder Technol. 2015, 277, 141–146. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of low molecular weight chitosan and chitooligosaccharides (COS): A review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Wang, S.T.; Tsai, C.C.; Shih, M.C.; Tsai, M.L. Flavor-related applications of chitin and chitosan in foods: Effect of Structure and Properties on the Efficacy. In Chitosan for Biomaterials III. Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Eds.; Springer: Cham, Switzerland, 2021; Volume 287, pp. 169–202. [Google Scholar]
- Yi, C.; Tsai, M.L.; Liu, T. Spray-dried chitosan/acid/NaCl microparticles enhance saltiness perception. Carbohydr. Polym. 2017, 172, 246–254. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Wang, S.T.; Shih, M.C.; Tsai, M.L. Chitosan/NaCl microparticles used as sodium reduction strategy: Studies on the physicochemical characteristics and optimization of preparation conditions. J. Fish. Soc. Taiwan 2019, 46, 65–77. [Google Scholar]
- Phan, V.A.; Yven, C.; Lawrence, G.; Chabanet, C.; Reparet, J.M.; Salles, C. In vivo sodium release related to salty perception during eating model cheeses of different textures. Int. Dairy J. 2008, 18, 956–963. [Google Scholar] [CrossRef]
- Mosca, A.C.; Andriot, I.; Guichard, E.; Salles, C. Binding of Na+ ions to proteins: Effect on taste perception. Food Hydrocoll. 2015, 51, 33–40. [Google Scholar] [CrossRef]
- Tsai, W.C.; Wang, S.T.; Chang, K.L.B.; Tsai, M.L. Enhancing saltiness perception using chitin nanomaterials. Polymers 2019, 11, 719. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.L.; Bai, S.W.; Chen, R.H. Cavitation effects versus stretch effects resulted in different size and polydispersity of ionotropic gelation chitosan–sodium tripolyphosphate nanoparticle. Carbohydr. Polym. 2008, 71, 448–457. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinamaki, J.; de la Paz, N.; Lopez, O.; Maunu, S.L.; Virtanen, T.; Hatanpää, T.; Antikainen, O.; Nogueira, A.; Fundora, J.; et al. Effects of spray drying on physicochemical properties of chitosan acid salts. AAPS PharmSciTech 2011, 12, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W. Characterization of spray-dried soy sauce powders using maltodextrins as carrier. J. Food Eng. 2012, 109, 399–405. [Google Scholar] [CrossRef]
- Levallois, B.; Fovet, Y.; Lapeyre, L.; Gal, J.Y. In vitro fluoride release from restorative materials in water versus artificial saliva medium (SAGF). Dent. Mater. 1998, 14, 441–447. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W. Characterization of spray dried soy sauce powders made by adding crystalline carbohydrates to drying carrier. Food Chem. 2015, 168, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Taip, F.S.; Aziz, N.A.; Talib, R.A. Physical properties of spray-dried pink guava (Psidium guajava) powder. Agric. Agric. Sci. Procedia 2014, 2, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Lebrun, P.; Krier, F.; Mantanus, J.; Grohganz, H.; Yang, M.; Rozet, E.; Boulanger, B.; Evrard, B.; Rantanen, J.; Hubert, P. Design space approach in the optimization of the spray-drying process. Eur. J. Pharm. Biopharm. 2012, 80, 226–234. [Google Scholar] [CrossRef]
- Wahl, M.; Bröckel, U.; Brendel, L.; Feise, H.J.; Weigl, B.; Röck, M.; Schwedes, J. Understanding powder caking: Predicting caking strength from individual particle contacts. Powder Technol. 2008, 188, 147–152. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Zhu, Y.; Hu, Y.; Li, C. Aerosol synthesis of graphene-Fe3O4 hollow hybrid microspheres for heterogeneous fenton and electro-fenton reaction. J. Environ. Chem. Eng. 2016, 4, 2469–2476. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Iqbal, T.; Delaney, C.; Twomey, T.; Keogh, M.K. Effect of powder properties and storage conditions on the flowability of milk powders with different fat contents. J. Food Eng. 2004, 64, 435–444. [Google Scholar] [CrossRef]
- Muzaffar, K.; Nayik, G.A.; Kumar, P. Stickiness problem associated with spray drying of sugar and acid rich foods: A mini review. J. Nutr. Food Sci. 2015, 5 (Suppl. S12), 3. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Effect of maltodextrin and gum arabic on water sorption and glass transition temperature of spray dried chicken meat hydrolysate protein. J. Food Eng. 2009, 91, 287–296. [Google Scholar] [CrossRef]
- Fongin, S.; Granados, A.E.A.; Harnkarnsujarit, N.; Hagura, Y.; Kawai, K. Effects of maltodextrin and pulp on the water sorption, glass transition, and caking properties of freeze-dried mango powder. J. Food Eng. 2019, 247, 95–103. [Google Scholar] [CrossRef]
- Azhar, M.D.; Abd Hashib, S.; Ibrahim, U.K.; Abd Rahman, N. Development of carrier material for food applications in spray drying technology: An overview. Mater. Today Proc. 2021, 47, 1371–1375. [Google Scholar] [CrossRef]
- Phisut, N. Spray drying technique of fruit juice powder: Some factors influencing the properties of product. Int. Food Res. 2012, 19, 1297–1306. [Google Scholar]
- Dong, Y.; Paukkonen, H.; Fang, W.; Kontturi, E.; Laaksonen, T.; Laaksonen, P. Entangled and colloidally stable microcrystalline cellulose matrices in controlled drug release. Int. J. Pharm. 2018, 548, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Tsai, M.L.; Liu, T. Chitin nanofiber as a promising candidate for improved salty taste. LWT 2017, 75, 65–71. [Google Scholar] [CrossRef]
- Hsueh, C.Y.; Tsai, M.L.; Liu, T. Enhancing saltiness perception using chitin nanofibers when curing tilapia fillets. LWT 2017, 86, 93–98. [Google Scholar] [CrossRef]
- Zhao, C.J.; Kinner, M.; Wismer, W.; Gänzle, M.G. Effect of glutamate accumulation during sourdough fermentation with Lactobacillus reuteri on the taste of bread and sodium-reduced bread. Cereal Chem. 2015, 92, 224–230. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Temme, E.H.; Koeman, F.T.; Noort, M.W.; Kremer, S.; Janssen, A.M. A salt reduction of 50% in bread does not decrease bread consumption or increase sodium intake by the choice of sandwich fillings. J. Nutr. 2011, 141, 2249–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, M.; Cavaleiro, C.; Ramos, F. Sodium reduction in bread: A role for glasswort (Salicornia ramosissima J. woods). Compr. Rev. Food Sci. Food Saf. 2017, 16, 1056–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| NaCl | Chitosan | Maltodextrin | Microcrystalline Cellulose | Waxy Maize Starch | |
|---|---|---|---|---|---|
| CNM | 30.6 | 4.3 | 65.1 | 0 | 0 |
| MCC10 | 27.2 | 3.8 | 58.0 | 11.0 | 0 |
| MCC15 | 25.5 | 3.7 | 54.5 | 16.3 | 0 |
| MCC20 | 23.9 | 3.5 | 51.0 | 21.6 | 0 |
| WS10 | 27.2 | 3.8 | 58.0 | 0 | 11.0 |
| WS15 | 25.5 | 3.7 | 54.5 | 0 | 16.3 |
| WS20 | 23.9 | 3.5 | 51.0 | 0 | 21.6 |
| Yield (%) | Moisture Content (%) | NaCl Content (%) | Acetic Acid Content (%) | |
|---|---|---|---|---|
| CNM | 55.65 | 7.37 ± 0.68 c | 27.69 ± 0.55 bc | 2.00 ± 0.28 a |
| MCC10 | 43.88 | 5.18 ± 0.90 a | 27.69 ± 0.55 bc | 2.40 ± 0.49 a |
| MCC15 | 43.60 | 5.04 ± 0.91 a | 28.86 ± 0.73 c | 2.20 ± 0.28 a |
| MCC20 | 44.80 | 6.12 ± 0.30 abc | 26.72 ± 0.28 ab | 2.10 ± 0.24 a |
| WS10 | 40.83 | 5.82 ± 0.32 ab | 28.47 ± 1.46 c | 1.60 ± 0.28 a |
| WS15 | 44.21 | 5.35 ± 0.47 ab | 27.69 ± 0.55 bc | 1.90 ± 0.14 a |
| WS20 | 39.29 | 6.68 ± 0.24 bc | 25.35 ± 0.28 a | 1.60 ± 0.28 a |
| Microparticles (μm) | NaCl Crystals (μm) | |
|---|---|---|
| CNM | 6.79 ± 2.57 a | 0.36 ± 0.12 a |
| MCC10 | 6.29 ± 1.97 a | 0.43 ± 0.11 ab |
| MCC15 | 6.94 ± 2.75 a | 0.48 ± 0.16 ab |
| MCC20 | 7.03 ± 3.37 a | 0.49 ± 0.13 b |
| WS10 | 7.64 ± 2.20 b | 1.24 ± 0.36 d |
| WS15 | 6.76 ± 2.58 a | 0.66 ± 0.25 c |
| WS20 | 7.02 ± 2.19 ab | 0.63 ± 0.19 c |
| Bulk Density (g/cm3) | Tapped Density (g/cm3) | Flowability | ||
|---|---|---|---|---|
| Hausner Ratio | Carr Index | |||
| CNM | 0.29 ± 0.02 b | 0.34 ± 0.01 a | 1.33 ± 0.03 ab | 24.68 ± 1.49 ab |
| MCC10 | 0.28 ± 0.00 b | 0.37 ± 0.01 ab | 1.19 ± 0.04 a | 15.60 ± 2.77 a |
| MCC15 | 0.29 ± 0.02 b | 0.36 ± 0.02 ab | 1.23 ± 0.00 ab | 18.90 ± 0.23 a |
| MCC20 | 0.32 ± 0.02 c | 0.40 ± 0.01 bc | 1.25 ± 0.08 ab | 19.90 ± 5.27 ab |
| WS10 | 0.24 ± 0.01 a | 0.35 ± 0.05 a | 1.43 ± 0.18 b | 28.87 ± 8.71 b |
| WS15 | 0.28 ± 0.01 b | 0.38 ± 0.01 abc | 1.37 ± 0.06 ab | 27.04 ± 3.25 b |
| WS20 | 0.34 ± 0.00 c | 0.42 ± 0.00 c | 1.24 ± 0.01 ab | 19.50 ± 0.70 b |
| Hygroscopic Rate 0–36 h (g/100 g·h) | Hygroscopic Rate 0–72 h (g/100 g·h) | Caking Strength (kg) | |
|---|---|---|---|
| CNM | 4.45 ± 0.14 c | 2.83 ± 0.10 c | 3.27 ± 0.21 ab |
| MCC10 | 3.80 ± 0.55 bc | 2.52 ± 0.17 b | 2.91 ± 0.21 bc |
| MCC15 | 3.94 ± 0.10 ab | 2.33 ± 0.16 a | 2.97 ± 0.29 ab |
| MCC20 | 3.74 ± 0.07 a | 2.33 ± 0.05 a | 2.57 ± 0.26 a |
| WS10 | 4.19 ± 0.10 bc | 2.59 ± 0.05 b | 4.42 ± 0.38 c |
| WS15 | 4.09 ± 0.11 b | 2.56 ± 0.11 b | 3.79 ± 0.20 bc |
| WS20 | 3.69 ± 0.28 a | 2.32 ± 0.12 a | 3.26 ± 0.28 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-T.; Lu, Y.-Y.; Tsai, M.-L. Formulation and Evaluation of Chitosan/NaCl/Maltodextrin Microparticles as a Saltiness Enhancer: Study on the Optimization of Excipients for the Spray-Drying Process. Polymers 2021, 13, 4302. https://doi.org/10.3390/polym13244302
Wang S-T, Lu Y-Y, Tsai M-L. Formulation and Evaluation of Chitosan/NaCl/Maltodextrin Microparticles as a Saltiness Enhancer: Study on the Optimization of Excipients for the Spray-Drying Process. Polymers. 2021; 13(24):4302. https://doi.org/10.3390/polym13244302
Chicago/Turabian StyleWang, Shang-Ta, Yi-Ying Lu, and Min-Lang Tsai. 2021. "Formulation and Evaluation of Chitosan/NaCl/Maltodextrin Microparticles as a Saltiness Enhancer: Study on the Optimization of Excipients for the Spray-Drying Process" Polymers 13, no. 24: 4302. https://doi.org/10.3390/polym13244302
APA StyleWang, S. -T., Lu, Y. -Y., & Tsai, M. -L. (2021). Formulation and Evaluation of Chitosan/NaCl/Maltodextrin Microparticles as a Saltiness Enhancer: Study on the Optimization of Excipients for the Spray-Drying Process. Polymers, 13(24), 4302. https://doi.org/10.3390/polym13244302