Features of Functionalization of the Surface of Alumina Nanofibers by Hydrolysis of Organosilanes on Surface Hydroxyl Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
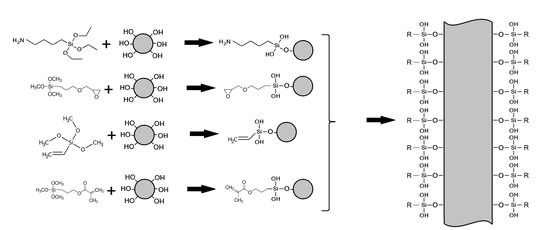
2.2. Silanization
2.3. Simultaneous Thermal Analysis
2.4. XPS
3. Results
3.1. Raw Alumina Nanofibers
3.2. Fixation of Functional Groups on the Surface of Alumina Nanofibers
3.3. Thermal Properties of Functionalized Alumina Nanofibers
3.4. Silanization Stoichiometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nadeem, B.; Irshad, K.; Wail, F. Nanomaterials: A review of synthesis methods, properties, recent progress, and Challenges. Mater. Adv. 2021, 1821–1871. [Google Scholar] [CrossRef]
- Saghafi, H.; Fotouhi, M.; Minak, G. Improvement of the Impact Properties of Composite Laminates by Means of Nano-Modification of the Matrix—A Review. Appl. Sci. 2018, 8, 2406. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Lim, J.-V.; Bee, S.-T.; Tin Sin, L.; Ratnam, C.T.; Abdul Hamid, Z.A. A Review on the Synthesis, Properties, and Utilities of Functionalized Carbon Nanoparticles for Polymer Nanocomposites. Polymers 2021, 13, 3547. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef]
- Embabi, M.; Kweon, M.S.; Chen, Z.; Lee, P.C. Tunable Tensile Properties of Polypropylene and Polyethylene Terephthalate Fibrillar Blends through Micro-/Nanolayered Extrusion Technology. Polymers 2020, 12, 2585. [Google Scholar] [CrossRef]
- Pras, M.; Gérard, J.-F.; Golanski, L.; Quintard, G.; Duchet-Rumeau, J. Key Role of the Dispersion of Carbon Nanotubes (CNTs) within Epoxy Networks on their Ability to Release. Polymers 2020, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- Saharudin, M.S.; Jumahat, A.; Kahar, A.Z.; Ahmad, S. The Influence of Alumina Filler on Impact Properties of Short Glass Fiber Reinforced Epoxy. Appl. Mech. Mater. 2013, 393, 88–93. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [Green Version]
- Pitsa, D.; Danikas, M.G. Interfaces features in polymer nanocomposites: A review of proposed models. NANO Brief Rep. Rev. 2011, 6, 497–508. [Google Scholar] [CrossRef]
- Connor, R.B.; Eileen, B.; Makoto, A.; Kai, Z.; Christopher, J.D.; Sanat, K.K.; Yucheng, H.; Brian, C.B.; David, W.G.; Shiwang, C.; et al. Polymer-Grafted Nanoparticle Membranes with Controllable Free Volume. Macromolecules 2017, 50, 7111–7120. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Surface Chemistry of Silanes at the Interface. Silane Coupling Agents; Springer: Boston, MA, USA, 1982. [Google Scholar] [CrossRef]
- Odalanowska, M.; Woźniak, M.; Ratajczak, I.; Zielińska, D.; Cofta, G.; Borysiak, S. Propolis and Organosilanes as Innovative Hybrid Modifiers in Wood-Based Polymer Composites. Materials 2021, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Pegg, E.C.; Walker, G.S.; Scotchford, C.A.; Farrar, D.; Grant, D. Mono-functional aminosilanes as primers for peptide functionalization. J. Biomed. Mater. Res. Part A 2009, 90A, 947–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, M.J.; Williams, D.E. Surface modification by fluoroalkyl-functional silanes. J. Adhes. Sci. Technol. 1991, 5, 307–320. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsieh, T.-E. The effect of organo-functional silanes on the adhesion of epoxide resins to ITO glass. J. Adhes. Sci. Technol. 2005, 1, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Frickel, N.; Messing, R.; Gelbrich, T.; Schmidt, A.M. Functional Silanes as Surface Modifying Primers for the Preparation of Highly Stable and Well-Defined Magnetic Polymer Hybrids. Langmuir 2010, 26, 2839–2846. [Google Scholar] [CrossRef]
- Kutuzov, M. Method and System for Alumina Nanofibers Synthesis from Molten Aluminum. ANF Technology Limited, assignee. U.S. Patent WO2013114183 A1, 8 August 2013. [Google Scholar]
- Kuular, A.A.; Simunin, M.M.; Bermeshev, T.V.; Voronin, A.S.; Dobrosmyslov, S.S.; Fadeev, Y.V.; Molokeev, M.S.; Volochaev, M.N.; Khartov, S.V. The Influence of Alumina Nanofibers on the Physical and Mechanical Properties of Mineral-Filled Polyethylene: An Experimental Study. Tech. Phys. Lett. 2020, 46, 1215–1218. [Google Scholar] [CrossRef]
- Kuular, A.A.; Voronin, A.S.; Markevich, I.A.; Bermeshev, T.V.; Simunin, M.M. Mechanical properties UHMWPE/alumina nanofibers nanocomposite. J. Phys. Conf. Ser. 2020, 1679, 042100. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization—The way for advanced applications of smart materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Wichaita, W.; Kim, Y.; Tangboriboonrat, P.; Thérien-Aubin, H. Polymer-functionalized polymer nanoparticles and their behaviour in suspensions. Polym. Chem. 2020, 11, 2119–2128. [Google Scholar] [CrossRef]
- Podkościelna, B.; Wawrzkiewicz, M.; Klapiszewski, Ł. Synthesis, Characterization and Sorption Ability of Epoxy Resin-Based Sorbents with Amine Groups. Polymers 2021, 13, 4139. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Vorob’eva, A.I.; Shimanovich, D.L.; Sycheva, O.A. Studying the Thermodynamic Characteristics of Anodic Alumina. Russ. Microelectron. 2018, 47, 40–49. [Google Scholar] [CrossRef]
- Crisan, M.; Jitianu, A.; Crisan, D.; Balasoiu, M.; Dragan, N.; Zaharescu, M. Sol-gel monocomponent nano-sized oxide powders. J. Optoelectron. Adv. Mater. 2000, 4, 339–344. [Google Scholar] [CrossRef]
- Aghayan, M.; Hussainova, I.; Gasik, M.; Kutuzov, M.; Friman, M. Coupled thermal analysis of novel alumina nanofibers with ultrahigh aspect ratio. Thermochim. Acta 2013, 574, 140–144. [Google Scholar] [CrossRef]
- Lara-Ochoa, F.; Silaghi-Dumitrescu, I.; Haiduc, I. Coordinative Dimerization of Aminosilanes. Model MNDO and Ab Initio Molecular Orbital Calculations. Main Group Chem. 1996, 4, 387–398. [Google Scholar] [CrossRef]




| Sample | C 1s | Al 2p | O 1s | Si 2p | N 1s |
|---|---|---|---|---|---|
| at. % | at. % | at. % | at. % | at. % | |
| ABES -NFA | 26.0 | 23.9 | 45.2 | 2.5 | 2.3 |
| MAMS-NFA | 32.5 | 19.5 | 46.5 | 1.5 | - |
| VTMS-NFA | 20.0 | 25.5 | 51.8 | 2.7 | - |
| GlyMS-NFA | 29.0 | 20.8 | 48.8 | 1.4 | - |
| Functional Group Names | Molecular Weight of Silane Primer | Molecular Weight of Linked Group | STA Weight Fraction of Group% | Stoichiometric Weight Fraction of Group% |
|---|---|---|---|---|
| Hydroxyl | 17.01 | 1.61 | ||
| Vinyl | 148.23 | 55.23 | 4.9 | 4.92 |
| Methacryl | 248.24 | 155.24 | 10.9 | 12.70 |
| Epoxypropyl | 236.34 | 143.34 | 11.9 | 11.84 |
| Aminobutyl | 235.40 | 100.40 | 12.3 | 8.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simunin, M.M.; Voronin, A.S.; Fadeev, Y.V.; Mikhlin, Y.L.; Lizunov, D.A.; Samoilo, A.S.; Chirkov, D.Y.; Voronina, S.Y.; Khartov, S.V. Features of Functionalization of the Surface of Alumina Nanofibers by Hydrolysis of Organosilanes on Surface Hydroxyl Groups. Polymers 2021, 13, 4374. https://doi.org/10.3390/polym13244374
Simunin MM, Voronin AS, Fadeev YV, Mikhlin YL, Lizunov DA, Samoilo AS, Chirkov DY, Voronina SY, Khartov SV. Features of Functionalization of the Surface of Alumina Nanofibers by Hydrolysis of Organosilanes on Surface Hydroxyl Groups. Polymers. 2021; 13(24):4374. https://doi.org/10.3390/polym13244374
Chicago/Turabian StyleSimunin, Mikhail M., Anton S. Voronin, Yurii V. Fadeev, Yurii L. Mikhlin, Denis A. Lizunov, Alexandr S. Samoilo, Dmitrii Yu. Chirkov, Svetlana Yu. Voronina, and Stas V. Khartov. 2021. "Features of Functionalization of the Surface of Alumina Nanofibers by Hydrolysis of Organosilanes on Surface Hydroxyl Groups" Polymers 13, no. 24: 4374. https://doi.org/10.3390/polym13244374
APA StyleSimunin, M. M., Voronin, A. S., Fadeev, Y. V., Mikhlin, Y. L., Lizunov, D. A., Samoilo, A. S., Chirkov, D. Y., Voronina, S. Y., & Khartov, S. V. (2021). Features of Functionalization of the Surface of Alumina Nanofibers by Hydrolysis of Organosilanes on Surface Hydroxyl Groups. Polymers, 13(24), 4374. https://doi.org/10.3390/polym13244374