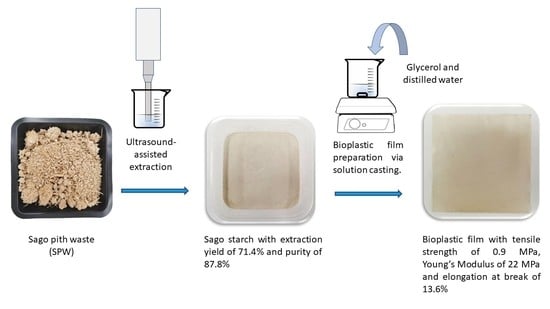
Rapid Ultrasound-Assisted Starch Extraction from Sago Pith Waste (SPW) for the Fabrication of Sustainable Bioplastic Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasound-Assisted Starch Extraction from SPW
2.3. Chemical Composition of Extracted Starch
2.3.1. Determination of Amylose Content
2.3.2. Determination of Starch Content
2.3.3. Protein Content
2.3.4. Determination of Fat Content
2.3.5. Determination of Ash Content
2.4. Determination of Extraction Yield and Extraction Efficiency
2.5. Preparation of Sago Starch Bioplastic Film
2.6. Characterization of Extracted Starch
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. Particle Size Distribution
2.6.3. X-ray Diffraction (XRD) Pattern
2.6.4. Solubility and Swelling Power
2.7. Characterization of Fabricated Bioplastic Films
2.7.1. Colour Properties
2.7.2. Tensile Test
2.7.3. Water Vapour Permeability (WVP)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Particle Size
3.2. Effect of Solid Loading
3.3. Effect of Sonication Duration and Ultrasonic Amplitude
3.4. Effect of Duty Cycle
3.5. Comparison between Conventional and Ultrasound-Assisted Extraction
3.6. Comparison of Optimum Reaction Conditions with Existing Literatures
3.7. Characterization of Extracted Starches
3.7.1. Surface Morphology
3.7.2. Particle Size Distribution
3.7.3. XRD
3.7.4. Swelling Power and Solubility
3.8. Characterization of Fabricated Bioplastic Films
3.8.1. Colour Properties
3.8.2. Mechanical Properties
3.8.3. Water Vapour Permeability (WVP)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.Y.; Lee, J.H.; Kim, J.Y.; Lim, W.J.; Lim, S.T. Characterization of nanoparticles prepared by acid hydrolysis of various starches. Starch-Stärke 2012, 64, 367–373. [Google Scholar] [CrossRef]
- Herrera, M.P.; Vasanthan, T. Rheological characterization of gum and starch nanoparticle blends. Food Chem. 2018, 243, 43–49. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lim, S.-T. Preparation of nano-sized starch particles by complex formation with n-butanol. Carbohydr. Polym. 2009, 76, 110–116. [Google Scholar] [CrossRef]
- Andrade, I.H.; Otoni, C.G.; Amorim, T.S.; Camilloto, G.P.; Cruz, R.S. Ultrasound-assisted extraction of starch nanoparticles from breadfruit (Artocarpus altilis (Parkinson) Fosberg). Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124277. [Google Scholar] [CrossRef]
- Song, D.; Thio, Y.S.; Deng, Y. Starch nanoparticle formation via reactive extrusion and related mechanism study. Carbohydr. Polym. 2011, 85, 208–214. [Google Scholar] [CrossRef]
- Minakawa, A.F.; Faria-Tischer, P.C.; Mali, S. Simple ultrasound method to obtain starch micro-and nanoparticles from cassava, corn and yam starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Kringel, D.H.; El Halal, S.L.M.; Zavareze, E.d.R.; Dias, A.R.G. Methods for the Extraction of Roots, Tubers, Pulses, Pseudocereals, and Other Unconventional Starches Sources: A Review. Starch-Stärke 2020, 72, 1900234. [Google Scholar] [CrossRef]
- Sit, N.; Misra, S.; Deka, S.C. Yield and functional properties of taro starch as affected by ultrasound. Food Bioproc. Tech. 2014, 7, 1950–1958. [Google Scholar] [CrossRef]
- Patel, C.M.; Chakraborty, M.; Murthy, Z. Fast and scalable preparation of starch nanoparticles by stirred media milling. Adv. Powder Technol. 2016, 27, 1287–1294. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, H.; Chang, P.R. Transitional properties of starch colloid with particle size reduction from micro-to nanometer. J. Colloid Interface Sci. 2009, 339, 117–124. [Google Scholar] [CrossRef]
- Polachini, T.C.; Hernando, I.; Mulet, A.; Telis-Romero, J.; Cárcel, J.A. Ultrasound-assisted acid hydrolysis of cassava (Manihot esculenta) bagasse: Kinetics, acoustic field and structural effects. Ultrason. Sonochem. 2021, 70, 105318. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Koubaa, M.; Barba, F.J.; Abedi, E.; Niakousari, M.; Tavakoli, J. Extraction of essential oil from Aloysia citriodora Palau leaves using continuous and pulsed ultrasound: Kinetics, antioxidant activity and antimicrobial properties. Process Biochem. 2018, 65, 197–204. [Google Scholar] [CrossRef]
- Liu, C.-W.; Wang, Y.-C.; Lu, H.-C.; Chiang, W.-D. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process Biochem. 2014, 49, 1601–1605. [Google Scholar] [CrossRef]
- Bernardo, C.O.; Ascheri, J.L.R.; Chávez, D.W.H.; Carvalho, C.W.P. Ultrasound assisted extraction of yam (Dioscorea bulbífera) starch: Effect on morphology and functional properties. Starch-Stärke 2018, 70, 1700185. [Google Scholar] [CrossRef]
- Karaman, M.; Tuncel, N.B.; Yılmaz Tuncel, N. The effect of ultrasound-assisted extraction on yield and properties of some pulse starches. Starch-Stärke 2017, 69, 1600307. [Google Scholar] [CrossRef]
- González-Lemus, L.B.; Calderón-Domínguez, G.; Salgado-Cruz, M.d.l.P.; Díaz-Ramírez, M.; Ramírez-Miranda, M.; Chanona-Pérez, J.J.; Gϋemes-Vera, N.; Farrera-Rebollo, R.R. Ultrasound-assisted extraction of starch from frozen jicama (P. erosus) roots: Effect on yield, structural characteristics and thermal properties. CyTA-J. Food 2018, 16, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.C.; Rahman, W.A.W.A.; Toh, W.Y. Characterisation of sago pith waste and its composites. Ind. Crops Prod. 2013, 45, 319–326. [Google Scholar] [CrossRef]
- Bukhari, N.A.; Loh, S.K.; Bakar, N.A.; Ismail, M. Hydrolysis of residual starch from sago pith residue and its fermentation to bioethanol. Sains Malays. 2017, 46, 1269–1278. [Google Scholar] [CrossRef]
- Husin, H.; Ibrahim, M.F.; Kamal Bahrin, E.; Abd-Aziz, S. Simultaneous saccharification and fermentation of sago hampas into biobutanol by Clostridium acetobutylicum ATCC 824. Energy Sci. Eng. 2019, 7, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Pinyo, J.; Luangpituksa, P.; Suphantharika, M.; Hansawasdi, C.; Wongsagonsup, R. Improvement of sago starch extraction process using various pretreatment techniques and their pretreatment combination. Starch-Stärke 2017, 69, 1700005. [Google Scholar] [CrossRef]
- Amin, N.; Sabli, N.; Izhar, S.; Yoshida, H. Sago Wastes and Its Applications. Pertanika J. Sci. Technol. 2019, 27, 1841–1862. [Google Scholar]
- Alonso-González, M.; Felix, M.; Romero, A. Development of malt sprout-based bioplastics via injection-moulding. Ind. Crops Prod. 2021, 162, 113267. [Google Scholar] [CrossRef]
- Jansens, K.J.; Hong, N.V.; Telen, L.; Brijs, K.; Lagrain, B.; Van Vuure, A.W.; Van Acker, K.; Verpoest, I.; Van Puyvelde, P.; Goderis, B. Effect of molding conditions and moisture content on the mechanical properties of compression molded glassy, wheat gluten bioplastics. Ind. Crops Prod. 2013, 44, 480–487. [Google Scholar] [CrossRef]
- Méité, N.; Konan, L.K.; Tognonvi, M.T.; Doubi, B.I.H.G.; Gomina, M.; Oyetola, S. Properties of hydric and biodegradability of cassava starch-based bioplastics reinforced with thermally modified kaolin. Carbohydr. Polym. 2020, 254, 117322. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Chowdhury, M.A.; Kowser, M.A. Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef] [Green Version]
- Santana, R.F.; Bonomo, R.C.F.; Gandolfi, O.R.R.; Rodrigues, L.B.; Santos, L.S.; dos Santos Pires, A.C.; de Oliveira, C.P.; Fontan, R.d.C.I.; Veloso, C.M. Characterization of starch-based bioplastics from jackfruit seed plasticized with glycerol. J. Food Sci. Technol. 2018, 55, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Agustin, M.B.; Ahmmad, B.; Alonzo, S.M.M.; Patriana, F.M. Bioplastic based on starch and cellulose nanocrystals from rice straw. J. Reinf. Plast. Compos. 2014, 33, 2205–2213. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.A.; Parimalavalli, R. Effect of starch isolation method on properties of sweet potato starch. Ann. Univ. Dunarea de Jos Galati Fascicle VI-Food Technol. 2014, 38, 48–63. [Google Scholar]
- Yoo, J.; Alavi, S.; Vadlani, P.; Amanor-Boadu, V. Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour. Technol. 2011, 102, 7583–7590. [Google Scholar] [CrossRef] [Green Version]
- Siljeström, M.; Westerlund, E.; Björck, I.; Holm, J.; Asp, N.-G.; Theander, O. The effects of various thermal processes on dietary fibre and starch content of whole grain wheat and white flour. J. Cereal Sci. 1986, 4, 315–323. [Google Scholar] [CrossRef]
- Dutta, H.; Paul, S.K.; Kalita, D.; Mahanta, C.L. Effect of acid concentration and treatment time on acid–alcohol modified jackfruit seed starch properties. Food Chem. 2011, 128, 284–291. [Google Scholar] [CrossRef]
- Vithu, P.; Dash, S.K.; Rayaguru, K.; Panda, M.K.; Nedunchezhiyan, M. Optimization of starch isolation process for sweet potato and characterization of the prepared starch. J. Food Meas. Charact. 2020, 14, 1520–1532. [Google Scholar] [CrossRef]
- Tejavathi, D.; Sujatha, B.; Karigar, C. Physicochemical properties of starch obtained from Curcuma karnatakensis-A new botanical source for high amylose content. Heliyon 2020, 6, e03169. [Google Scholar] [CrossRef] [Green Version]
- Klang, V.; Schwarz, J.C.; Matsko, N.; Rezvani, E.; El-Hagin, N.; Wirth, M.; Valenta, C. Semi-solid sucrose stearate-based emulsions as dermal drug delivery systems. Pharmaceutics 2011, 3, 275–306. [Google Scholar] [CrossRef] [Green Version]
- Kyriakidou, A.; Makris, D.P.; Lazaridou, A.; Biliaderis, C.G.; Mourtzinos, I. Physical Properties of Chitosan Films Containing Pomegranate Peel Extracts Obtained by Deep Eutectic Solvents. Foods 2021, 10, 1262. [Google Scholar] [CrossRef]
- ASTM. Standard test method for tensile properties of thin plastic sheeting-D882-02. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2002; pp. 1–9. [Google Scholar]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Goula, A.M. Ultrasound-assisted extraction of pomegranate seed oil–Kinetic modeling. J. Food Eng. 2013, 117, 492–498. [Google Scholar] [CrossRef]
- Rozalli, N.M.; Chin, N.; Yusof, Y. Grinding characteristics of Asian originated peanuts (Arachishypogaea L.) and specific energy consumption during ultra-high speed grinding for natural peanut butter production. J. Food Eng. 2015, 152, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lee, Y.J.; Yoon, W.B. Effect of moisture content on the grinding process and powder properties in food: A review. Processes 2018, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.X.; Ong, H.C.; Lim, S.; Pang, Y.L.; Milano, J. Process intensification of biodiesel synthesis via ultrasound-assisted in situ esterification of Jatropha oil seeds. J. Chem. Technol. Biotechnol. 2019, 94, 1362–1373. [Google Scholar] [CrossRef]
- Shirsath, S.; Sable, S.; Gaikwad, S.; Sonawane, S.; Saini, D.; Gogate, P. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M. Preparation of nanoparticle dispersions from powdered material using ultrasonic disruption. NIST Spec. Publ. 2012, 1200, 1–15. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Patil, S.S.; Pathak, A.; Rathod, V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021, 70, 105267. [Google Scholar] [CrossRef] [PubMed]
- Nitayavardhana, S.; Rakshit, S.K.; Grewell, D.; Van Leeuwen, J.; Khanal, S.K. Ultrasound pretreatment of cassava chip slurry to enhance sugar release for subsequent ethanol production. Biotechnol. Bioeng. 2008, 101, 487–496. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Liu, Y.; Wu, Y.; Wu, Z. Ultrasound extraction of polysaccharides from guava leaves and their antioxidant and antiglycation activity. Process Biochem. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef]
- Vasudeo, C.G. Optimization of Ultrasound-Assisted Extraction (UAE) of Starch from Cassava (Manihot esculenta Grantz) using Response Surface Methodology (RSM) Technique; ICAR-CTCRI: Tamil Nadu, India, 2016. [Google Scholar]
- Patil, S.S.; Rathod, V.K. Synergistic effect of ultrasound and three phase partitioning for the extraction of curcuminoids from Curcuma longa and its bioactivity profile. Process Biochem. 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gogate, P.R. Intensifying the biogas production from food waste using ultrasound: Understanding into effect of operating parameters. Ultrason. Sonochem. 2019, 59, 104755. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. Intensification of bio-actives extraction from pomegranate peel using pulsed ultrasound: Effect of factors, correlation, optimization and antioxidant bioactivities. Ultrason. Sonochem. 2020, 72, 105423. [Google Scholar] [CrossRef]
- Mujtaba, M.; Masjuki, H.; Kalam, M.; Ong, H.C.; Gul, M.; Farooq, M.; Soudagar, M.E.M.; Ahmed, W.; Harith, M.; Yusoff, M. Ultrasound-assisted process optimization and tribological characteristics of biodiesel from palm-sesame oil via response surface methodology and extreme learning machine-Cuckoo search. Renew. Energy 2020, 158, 202–214. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, W.; Zhang, S.; Li, B.; Li, J. Evolution of solidification structures and mechanical properties of high-Si Al alloys under permanent magnetic stirring. Mater. Charact. 2019, 157, 109894. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Wan, N.; Wang, X.; Yang, M. Extraction of high-amylose starch from Radix Puerariae using high-intensity low-frequency ultrasound. Ultrason. Sonochem. 2019, 59, 104710. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-T.; Bhat, R.; Karim, A.A. Effects of sodium dodecyl sulphate and sonication treatment on physicochemical properties of starch. Food Chem. 2010, 120, 703–709. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, Y.; Eckhoff, S.R.; Feng, H. Sonication enhanced cornstarch separation. Starch-Stärke 2005, 57, 240–245. [Google Scholar] [CrossRef]
- Park, S.H.; Bean, S.; Wilson, J.; Schober, T. Rapid isolation of sorghum and other cereal starches using sonication. Cereal Chem. 2006, 83, 611–616. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Xu, F.; He, S.; Zhang, Y.; Sun, L.; Zhu, K.; Li, S.; Wu, G.; Tan, L. Jackfruit starch: Composition, structure, functional properties, modifications and applications. Trends Food Sci. Technol. 2020, 107, 268–283. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, C.; Feng, Y.; Zhang, J.; He, Y.; Duan, Y.; Zhang, H.; Ma, H. Effects of ultrasound-assisted extraction on the structural, functional and antioxidant properties of Dolichos lablab L. Protein. Process Biochem. 2021, 101, 274–284. [Google Scholar] [CrossRef]
- Seo, H.-W.; Kim, J.-H. Ultrasound-assisted fractional precipitation of paclitaxel from Taxus chinensis cell cultures. Process Biochem. 2019, 87, 238–243. [Google Scholar] [CrossRef]
- Borah, P.P.; Das, P.; Badwaik, L.S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochem. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on supramolecular structural changes of ultrasonic treated potato starch granules. Food Hydrocoll. 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Manchun, S.; Nunthanid, J.; Limmatvapirat, S.; Sriamornsak, P. Effect of Ultrasonic Treatment on Physical Properties of Tapioca Starch. Adv. Mater. Res. 2012, 506, 294–297. [Google Scholar] [CrossRef]
- Ali, M.A.; Razafindralambo, H.L.; Conti, G.; De Coninck, J.l. Bulk and Surface Wettability Characteristics of Probiotic Powders in Their Compressed Disc and Packed-Bed Column Forms. ACS Omega 2020, 5, 22348–22355. [Google Scholar] [CrossRef]
- Polnaya, F.; Marseno, D.; Cahyanto, M. Effects of phosphorylation and cross-linking on the pasting properties and molecular structure of sago starch. Int. Food Res. J. 2013, 20, 1609–1615. [Google Scholar]
- Uthumporn, U.; Wahidah, N.; Karim, A. Physicochemical properties of starch from sago (Metroxylon Sagu) palm grown in mineral soil at different growth stages. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; p. 012026. [Google Scholar]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Sousa, A.M.; Hilliou, L.; Gonçalves, M.P.; Souza, H.K. Thermo-compression molding of chitosan with a deep eutectic mixture for biofilms development. Green Chem. 2016, 18, 1571–1580. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef] [PubMed]








| Parameters | Ultrasound | Conventional (Magnetic Stirring) |
|---|---|---|
| Extraction yield (%) | 71.4 ± 0.4 a | 60.9 ± 1.2 b |
| Extraction efficiency (%) | 89.7 ± 0.5 a | 77.8 ± 1.5 b |
| Reaction time (min) | 5 a | 30 b |
| Amylose content (%) | 32.1 ± 0.0 a | 37.4 ± 0.0 b |
| Starch content (%) | 87.8 ± 0.1 a | 89.3 ± 0.1 b |
| Protein content (%) | 0.1 ± 0.0 a | 0.47 ± 0.1 b |
| Ash content (%) | 0.7 ± 0.1 a | 0.9 ± 0.0 b |
| Fat content (%) | n.d. | n.d. |
| Feedstock | Particle Size (µm) | Solid Loading (wt.%) | Sonication Duration (min) | Ultrasonic Amplitude (%) | Duty Cycle (%) | Extraction Yield (%) | Purity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SPW | Smaller than 250 | 10 | 5 | 70 | 83.3 | 71.4 | 87.8 | Present study |
| Sago pith | - | 10 | 7 | 80 | - | 71.5 | 95.0 | [20] |
| Yam tuber | - | 50 | 15 a | 70 a | - | 32.1 | - | [14] |
| Jicama roots | - | 25 | 10 a | - | - | 24.0 | 80.9 | [16] |
| Taro | - | 50 | 10 a | 50 a | 50 a | 19.0 * | - | [8] |
| Film Fabricated Using | Control Starch | Ultrasound-Extracted Starch | |
|---|---|---|---|
| Colour properties | L | 84.4 ± 0.4 a | 80.9 ± 0.0 b |
| a | 1.14 ± 0.0 a | 1.13 ± 0.0 a | |
| b | 12.1 ± 0.1 a | 11.9 ± 0.0 b | |
| 13.7 ± 0.0 a | 15.6 ± 0.1 b | ||
| Mechanical properties | Tensile strength (MPa) | 0.9 ± 0.3 a | 0.9 ± 0.1 a |
| Young’s Modulus (MPa) | 22.6 ± 1.1 a | 22.0 ± 0.8 a | |
| Elongation at break (%) | 13.8 ± 1.8 a | 13.6 ± 2.0 a | |
| Barrier property | WVP × 10−8 (g m−1 s−1 Pa−1) | 1.13 ± 0.4 a | 1.11 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.X.; Andriyana, A.; Lim, S.; Ong, H.C.; Pang, Y.L.; Ngoh, G.C. Rapid Ultrasound-Assisted Starch Extraction from Sago Pith Waste (SPW) for the Fabrication of Sustainable Bioplastic Film. Polymers 2021, 13, 4398. https://doi.org/10.3390/polym13244398
Tan SX, Andriyana A, Lim S, Ong HC, Pang YL, Ngoh GC. Rapid Ultrasound-Assisted Starch Extraction from Sago Pith Waste (SPW) for the Fabrication of Sustainable Bioplastic Film. Polymers. 2021; 13(24):4398. https://doi.org/10.3390/polym13244398
Chicago/Turabian StyleTan, Shiou Xuan, Andri Andriyana, Steven Lim, Hwai Chyuan Ong, Yean Ling Pang, and Gek Cheng Ngoh. 2021. "Rapid Ultrasound-Assisted Starch Extraction from Sago Pith Waste (SPW) for the Fabrication of Sustainable Bioplastic Film" Polymers 13, no. 24: 4398. https://doi.org/10.3390/polym13244398
APA StyleTan, S. X., Andriyana, A., Lim, S., Ong, H. C., Pang, Y. L., & Ngoh, G. C. (2021). Rapid Ultrasound-Assisted Starch Extraction from Sago Pith Waste (SPW) for the Fabrication of Sustainable Bioplastic Film. Polymers, 13(24), 4398. https://doi.org/10.3390/polym13244398