Sensing Cd(II) Using a Disposable Optical Sensor Based on a Schiff Base Immobilisation on a Polymer-Inclusion Membrane. Applications in Water and Art Paint Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
2.3. Membrane Preparation
2.4. Analytical Procedure for Optical Sensing of Cd(II) Ions
2.5. Real Samples Preparation
- (a)
- Spiked groundwaterGroundwater-certified reference material (BCR®-610) (Joint Research Centre (JRC), Institute for Reference Materials and Measurements (IRMM), with a certified value for Cd of 2.94 ± 0.08 μg kg−1) was directly used for assessing the method performance.
- (b)
- Art-paint samplesTwo school acrylic art-paint samples were analysed: “cadmium yellow orange” (P020 colour index) and “cadmium red deep” (PR108 colour index). For that, 0.1 g of each paint were digested using 1 mL of HNO3 (65%), 1 mL of H2O2 (30%), and 1 mL of HClO4 (70%) in a Teflon vessel (waiting 24 h between the addition of each reagent (HNO3, H2O2, and HClO4); vessels were kept closed during that time). Two steps were followed for the digestion of the sample in a microwave oven at these conditions: 800 W, 120 °C for 10 min and 800 W, 170 °C for 30 min. The solution was made up to 25 mL with ultrapurified water. The final solutions for both art paints were diluted to obtain four different samples called acrylic paint 1, 2, 3, and 4, where 1 and 2 were two different dilutions from “yellow cadmium orange” paint and 3 and 4 were two different dilutions from “dark red cadmium” paint. These four samples were analysed following the new methodology proposed in this work for the determination of Cd(II) ions.
3. Results
3.1. Optimisation of the Optical Sensor Composition and Curing Time
3.2. Lifetime of the Optical Sensor
3.3. Effect of pH, Buffer Concentration, and Ionic Strength on the Response of the Sensor
3.4. Response Time of the Optical Sensor
3.5. Short-Term Stability
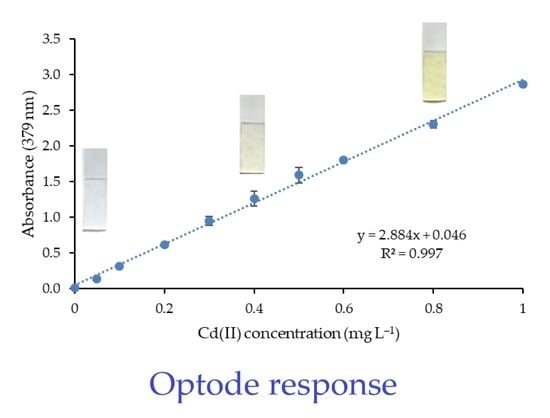
3.6. Analytical Performance of the Method
3.7. Analytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Time (h) | Replicate | Absorbance (324 nm) | Absorbance Average |
|---|---|---|---|
| 6 | 1 | 2.9883 | 2.9789 |
| 2 | 2.9694 | ||
| 12 | 1 | 2.9872 | 2.9791 |
| 2 | 2.9709 | ||
| 24 | 1 | 2.8851 | 2.9310 |
| 2 | 2.9769 | ||
| 30 | 1 | 2.9916 | 2.9846 |
| 2 | 2.9775 | ||
| 48 | 1 | 3.0012 | 2.9579 |
| 2 | 2.9146 | ||
| 96 | 1 | 2.9864 | 2.9937 |
| 2 | 3.0009 | ||
| 168 | 1 | 3.0087 | 3.0105 |
| 2 | 3.0123 |

References
- Ojeda, C.B.; Rojas, F.S. Recent development in optical chemical sensors coupling with flow infection analysis. Sensors 2006, 6, 1245–1307. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhou, X.; Su, F.; Tian, Y.; Ashili, S.; Holl, M.R.; Meldrum, D.R. Micro-patterning and characterization of PHEMA-co-PAM-based optical chemical sensors for lab-on-a-chip applications. Sens. Actuators B-Chem. 2012, 173, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Dashtian, K.; Zare-Dorabei, R. Preparation and characterization of a novel optical chemical sensor for determination of trace amounts of Praseodymium ion by UV/Vis spectrophotometry. Sens. Actuators B-Chem. 2017, 242, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Feng, J.; Hu, X.; Jia, C.; Huang, X. High sensitivity fiber sensor for measurement of Cd2+; concentration in aqueous solution based on reflective Mach-Zehnder interference with temperature calibration. Opt. Exp. 2019, 22, 32621–32629. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef]
- Kolev, S.D.; Almeida, M.I.G.S.; Cattrall, R.W. Polymer Inclusion Membranes: Smart materials for sensing and separation. In Handbook of Smart Materials in Analytical Chemistry, 1st ed.; de La Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; Volume 1, pp. 439–461. [Google Scholar]
- Suah, F.B.M. Preparation and characterization of a novel Co(II) optode based on polymer inclusion membrane. Anal. Chem. Res. 2017, 12, 40–46. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Chen, J.; Liu, Q.; Zeng, H. New insights into the interfacial behavior and swelling of polymer inclusion membrane (PIM) during Zn (II) extraction process. Chem. Eng. Sci. 2020, 220, 115620. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, H.; Hu, F.; Tang, J.; Yang, C.; Zhou, Y.; Lin, X.; Hu, J. Simultaneous recovery of copper(II) from two different feed solutions based on a three-compartment module with selective polymer inclusion membranes. Hydrometallurgy 2019, 188, 64–72. [Google Scholar] [CrossRef]
- Elias, G.; Díez, S.; Fontàs, C. System for mercury preconcentration in natural waters based on a polymer inclusion membrane incorporating an ionic liquid. J. Hazard. Mater. 2019, 371, 316–322. [Google Scholar] [CrossRef]
- Anticó, E.; Fontàs, C.; Vera, R.; Mostazo, G.; Salvadó, V.; Guasch, H. A novel Cyphos IL 104-based polymer inclusion membrane (PIM) probe to mimic biofilm zinc accumulation. Sci. Total Environ. 2020, 715, 136938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Albarrán, R.; de Gyves, J.; de San Miguel, E.R. Influence of some physicochemical parameters on the passive sampling of copper (II) from aqueous medium using a polymer inclusion membrane device. Environ. Pollut. 2020, 258, 113474. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Carner, C.A.; Croft, C.F.; Kolev, S.D.; Almeida, M.I.G.S. Green solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. [Google Scholar] [CrossRef]
- Ngarisan, N.I.; Ngah, C.W.Z.C.W.; Ahmad, M.; Kuswandi, B. Optimization of polymer inclusion membranes (PIMs) preparation for immobilization of Chrome Azurol S for optical sensing of aluminum(III). Sens. Actuators B-Chem. 2014, 203, 465–470. [Google Scholar] [CrossRef]
- Maiphetlho, K.; Chimuka, L.; Tutu, H.; Richards, H. Technical design and optimization of polymer inclusion membranes (PIMs) for sample pre-treatment and passive sampling—A review. Sci. Total Environ. 2021, 799, 149483. [Google Scholar] [CrossRef]
- Rezaei, B.; Hadadzadeh, H.; Azimi, A. Fabrication of an optical sensor based on the immobilization of Qsal on the plasticized PVC membrane for the determination of copper (II). J. Anal. Chem. 2012, 67, 687–693. [Google Scholar] [CrossRef]
- Ertekin, K.; Oter, O.; Ture, M.; Denizalti, S.; Cetinkaya, E. A long wavelength excitable fluorophore; chloro phenyl imino propenyl aniline (CPIPA) for selective sensing of Hg (II). J. Fluoresc. 2010, 20, 533–540. [Google Scholar] [CrossRef]
- Scindia, Y.M.; Pandey, A.K.; Reddy, A.V.R.; Manohar, S.B. Chemically selective membrane optode for Cr(VI) determination in aqueous samples. Anal. Chim. Acta 2004, 515, 311–321. [Google Scholar] [CrossRef]
- Casanueva-Marenco, M.J.; Díaz-de-Alba, M.; Herrera-Armario, A.; Galindo-Riaño, M.D.; Granado-Castro, M.D. Design and optimization of a single-use optical sensor based on a polymer inclusion membrane for zinc determination in drinks, food supplement and foot health care products. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 110, 110680. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, X.; Long, Y.; Chen, J.; Wang, L.; Zhang, Y. Optical sensing membrane for determination of trace cadmium(II), zinc(II) and copper(II) based on immobilization of 1-(2-pyridylazo)-2-naphthol on polymer inclusion membrane. Microchem. J. 2021, 162, 105767. [Google Scholar] [CrossRef]
- Coldur, M.; Oguzlar, S.; Ongun, M.Z.; Oter, O.; Yildirim, S. Usage of thiocyanate-based ionic liquid as new optical sensor reagent: Absorption and emission based selective determination of Fe (III) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117385. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M.; Heng, L.Y. Highly sensitive fluorescence optode for aluminium(III) based on non-plasticized polymer inclusion membrane. Sens. Actuators B-Chem. 2014, 201, 490–495. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M. Preparation and characterization of polymer inclusion membrane based optode for determination of Al3+ ion. Anal. Chim. Acta 2017, 951, 133–139. [Google Scholar] [CrossRef]
- Sharifi, H.; Tashkhourian, J.; Hemmateenejad, B. A 3D origami paper-based analytical device combined with PVC membrane for colorimetric assay of heavy metal ions: Application to determination of Cu(II) in water samples. Anal. Chim. Acta 2020, 1126, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Phichi, M.; Imyim, A.; Tuntulani, T.; Aeungmaitrepirom, W. Paper-based cation-selective optode sensor containing benzothiazole caliz [4]arene for dual colorimetric Ag+ and Hg2+ detection. Anal. Chim. Acta 2020, 1104, 147–155. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Spectrophotometric reagents. In Separation, Preconcentration and Spectrophotometry in Inorganic Analysis, 1st ed.; Elsevier: Amsterdam, The Netherland, 2000; pp. 53–73. [Google Scholar]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Volesky, B. Detoxification of metal-bearing effluents: Biosorption for the next century. Hydrometallurgy 2001, 59, 2003–2216. [Google Scholar] [CrossRef]
- Langmuir, D.; Chrostowski, P.; Chaney, R.; Vigneault, B. Issue paper on the environmental chemistry of metals. In US EPA Archive Document; Environmental Protection Agency Risk Assessment Forum: Washington, DC, USA, 2003. [Google Scholar]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef]
- McDonald, S.; Cresswell, T.; Hassell, K. Bioaccumulation kinetics of cadmium and zinc in the freshwater decapod crustacean Paratya australiensis following multiple pulse exposures. Sci. Total Environ. 2020, 720, 137609. [Google Scholar] [CrossRef]
- Figueira, E.; Lima, A.; Branco, D.; Quintino, V.; Rodrigues, A.M.; Freitas, R. Health concerns of consuming cockles (Cerastoderma edule L.) from a low contaminated coastal system. Environ. Int. 2011, 37, 956–972. [Google Scholar] [CrossRef] [PubMed]
- REGLAMENTO (UE) No 488/2014 DE LA COMISIÓN de 12 de mayo de 2014 que Modifica el Reglamento (CE) no 1881/2006 por lo que Respecta al Contenido Máximo de Cadmio en los Productos Alimenticios. Available online: https://www.boe.es/doue/2014/138/L00075-00079.pdf (accessed on 1 November 2021).
- Choi, W.J.; Kang, S.K.; Ham, S.; Chung, W.; Kim, A.J.; Kang, M. Chronic cadmium intoxication and renal injury among workers of a small-scale silver soldering company. Saf. Health Work 2020, 11, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Washington, DC, USA, 2012. [PubMed]
- Gardener, H.; Bowen, J.; Callan, S.P. Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Sci. Total Environ. 2019, 651, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Zvěřina, O.; Kuta, J.; Coufalík, P.; Kosečková, P.; Komárek, J. Simultaneous determination of cadmium and iron in different kinds of cereal flakes using high-resolution continuum source atomic absorption spectrometry. Food Chem. 2019, 298, 125084. [Google Scholar] [CrossRef]
- O’Mara, K.; Adams, M.; Burford, M.A.; Fry, B.; Cresswell, T. Uptake and accumulation of cadmium, manganese and zinc by fisheries species: Trophic differences in sensitivity to environmental metal accumulation. Sci. Total Environ. 2019, 690, 867–877. [Google Scholar] [CrossRef]
- Jain, R.B. Concentrations of cadmium, lead, and mercury in blood among US cigarettes, cigars, electronic cigarettes, and dual cigarette-e-cigarette users. Environ. Pollut. 2019, 251, 970–974. [Google Scholar] [CrossRef]
- Baloch, S.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Arain, M.B. Occupational exposure of lead and cadmium on adolescent and adult workers of battery recycling and welding workshops: Adverse impact on health. Sci. Total Environ. 2020, 720, 137549. [Google Scholar] [CrossRef]
- Turner, A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019, 657, 1409–1418. [Google Scholar] [CrossRef]
- Jha, R.; Dhanunjaya, R.; Meshram, A.; Verma, H.R.; Singh, K.K. Potential of polymer inclusion membrane process for selective recovery of metal values from waste printed circuit boards: A review. J. Clean Prod. 2020, 265, 121621. [Google Scholar] [CrossRef]
- Motsoane, N.; Maiphetlho, K.; Ncube, S.; Richards, H.; Kotze, I.; Tutu, H.; Cukrowska, E.; Chimuka, L. Technical development and optimisation of a passive sampler based on polymer inclusion membrane for uptake of copper, nickel, cobalt and cadmium in surface waters. Environ. Technol. Innov. 2020, 19, 100939. [Google Scholar] [CrossRef]
- Annane, K.; Sahmoune, A.; Montels, P.; Tingry, S. Polymer inclusion membrane extraction of cadmium(II) with Aliquat 336 in micro-channel cell. Chem. Eng. Res. Des. 2015, 94, 605–610. [Google Scholar] [CrossRef]
- Sanchez-Pedreño, C.; García, M.S.; Ortuño, J.A.; Albero, M.I.; Expósito, R. Kinetic methods for the determination of cadmium(II) based on a flow-through bulk optode. Talanta 2002, 56, 481–489. [Google Scholar] [CrossRef]
- Tharakeswar, Y.; Kalyan, Y.; Gangadhar, B.; Dumar, K.S.; Naidu, G.R. Optical chemical sensor for screening cadmium(II) in natural waters. J. Sens. Technol. 2012, 2, 68–74. [Google Scholar] [CrossRef] [Green Version]
- García-Vargas, M.; Bautista, J.M.; De Toro, P. Analytical possibilities of pyridine-2-acetaldehyde benzoylhydrazone as a chromogenic reagent. Microchem. J. 1981, 26, 557–568. [Google Scholar] [CrossRef]
- Zidan, A.S.A. Mixed ligand complexes of nickel (II) dialkyldithiophosphates with 2-acetylpyridine semicarbazone and 2-acetylpyridine benzoylhydrazone. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 567–582. [Google Scholar] [CrossRef]
- Granado-Castro, M.D.; Galindo-Riaño, M.D.; Domínguez-Lledó, F.C.; Díaz-López, C.; García-Vargas, M. Study of the kinetics of the transport of Cu(II), Cd(II) and Ni(II) ions through a liquid membrane. Anal. Bioanal. Chem. 2008, 391, 779–788. [Google Scholar] [CrossRef]









| Variable | Level | ||
|---|---|---|---|
| Lower Level (−1) | Central Level (0) | Upper Level (+1) | |
| PVC (g) | 2 | 2.5 | 3 |
| TBP (mL) | 3 | 4 | 5 |
| 2-APBH (g) | 0.02 | 0.03 | 0.04 |
| Replicates of Composition | Experiment | PVC (g) | TBP (mL) | 2-APBH (g) | Abs (379 nm) | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| 1 | 1 | −1 | −1 | −1 | 2.038 | 0.119 |
| 1 | 2 | −1 | 0 | 1 | 1.682 | 0.534 |
| 1 | 3 | −1 | 1 | 0 | 1.536 | 0.005 |
| 1 | 4 | 0 | −1 | 1 | 1.880 | 0.044 |
| 1 | 5 | 0 | 0 | 0 | 1.695 | 0.059 |
| 1 | 6 | 0 | 1 | −1 | 1.691 | 0.011 |
| 1 | 7 | 1 | −1 | 0 | 2.117 | 0.008 |
| 1 | 8 | 1 | 0 | −1 | 1.712 | 0.009 |
| 1 | 9 | 1 | 1 | 1 | 1.601 | 0.006 |
| 1 | 10 | 0 | 0 | 0 | 1.826 | 0.421 |
| 1 | 11 | 0 | 0 | 0 | 1.488 | 0.016 |
| 2 | 12 | −1 | −1 | −1 | 1.892 | 0.142 |
| 2 | 13 | −1 | 0 | 1 | 1.321 | 0.149 |
| 2 | 14 | −1 | 1 | 0 | 1.694 | 0.128 |
| 2 | 15 | 0 | −1 | 1 | 1.962 | 0.232 |
| 2 | 16 | 0 | 0 | 0 | 2.036 | 0.159 |
| 2 | 17 | 0 | 1 | −1 | 1.477 | 0.178 |
| 2 | 18 | 1 | −1 | 0 | 1.846 | 0.172 |
| 2 | 19 | 1 | 0 | −1 | 1.832 | 0.510 |
| 2 | 20 | 1 | 1 | 1 | 1.591 | 0.200 |
| 2 | 21 | 0 | 0 | 0 | 1.609 | 0.304 |
| 2 | 22 | 0 | 0 | 0 | 1.697 | 0.050 |
| Sample | (Cd(II)) ± SD (mg L−1) | tcalc | ttab (95%) | |
|---|---|---|---|---|
| Optical Sensor | AAS | |||
| Spiked groundwater with 0.3 mg L−1 | 0.309 ± 0.011 | 0.300 ± 0.002 | 1.440 | 3.182 |
| Spiked groundwater with 0.8 mg L−1 | 0.802 ± 0.001 | 0.806 ± 0.002 | 2.425 | 3.182 |
| Acrylic paint 1 | 0.544 ± 0.003 | 0.538 ± 0.009 | 0.878 | 3.182 |
| Acrylic paint 2 | 0.680 ± 0.013 | 0.664 ± 0.002 | 2.237 | 3.182 |
| Acrylic paint 3 | 0.478 ± 0.001 | 0.485 ± 0.004 | 1.935 | 3.182 |
| Acrylic paint 4 | 0.732 ± 0.047 | 0.743 ± 0.001 | 0.436 | 3.182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ponce, L.; Galindo-Riaño, M.D.; Casanueva-Marenco, M.J.; Granado-Castro, M.D.; Díaz-de-Alba, M. Sensing Cd(II) Using a Disposable Optical Sensor Based on a Schiff Base Immobilisation on a Polymer-Inclusion Membrane. Applications in Water and Art Paint Samples. Polymers 2021, 13, 4414. https://doi.org/10.3390/polym13244414
Sánchez-Ponce L, Galindo-Riaño MD, Casanueva-Marenco MJ, Granado-Castro MD, Díaz-de-Alba M. Sensing Cd(II) Using a Disposable Optical Sensor Based on a Schiff Base Immobilisation on a Polymer-Inclusion Membrane. Applications in Water and Art Paint Samples. Polymers. 2021; 13(24):4414. https://doi.org/10.3390/polym13244414
Chicago/Turabian StyleSánchez-Ponce, Lorena, María Dolores Galindo-Riaño, María José Casanueva-Marenco, María Dolores Granado-Castro, and Margarita Díaz-de-Alba. 2021. "Sensing Cd(II) Using a Disposable Optical Sensor Based on a Schiff Base Immobilisation on a Polymer-Inclusion Membrane. Applications in Water and Art Paint Samples" Polymers 13, no. 24: 4414. https://doi.org/10.3390/polym13244414
APA StyleSánchez-Ponce, L., Galindo-Riaño, M. D., Casanueva-Marenco, M. J., Granado-Castro, M. D., & Díaz-de-Alba, M. (2021). Sensing Cd(II) Using a Disposable Optical Sensor Based on a Schiff Base Immobilisation on a Polymer-Inclusion Membrane. Applications in Water and Art Paint Samples. Polymers, 13(24), 4414. https://doi.org/10.3390/polym13244414