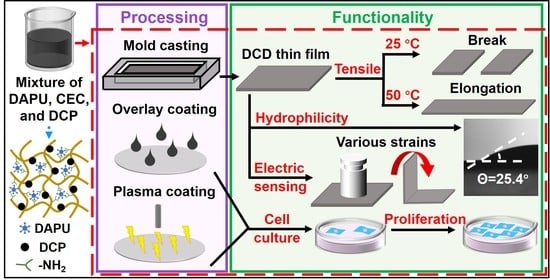
Thermoresponsive and Conductive Chitosan-Polyurethane Biocompatible Thin Films with Potential Coating Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Waterborne Synthesis of Dialdehyde Polyurethane Crosslinker (DAPU)
2.3. Synthesis of N-Carboxyethyl Chitosan (CEC)
2.4. Synthesis of Polypyrrole Modified with Double-Bonded Chitosan (DCP)
2.5. Preparation of DAPU/CEC/DCP Thin Films (DCDFs)
2.6. Physico-Chemical Characterization of DCDFs
2.7. Strain Sensing Function of DCDFs
2.8. Cell Attachment and Proliferation Analysis
2.9. Gene Expression of Neural-Related Marker for NSCs on DCDFs
2.10. Evaluation of DAPU/CEC/DCP (DCD) Materials as a Conductive Coating
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Optimization of DCDFs
3.2. Physico-Chemical Properties of Conductive DCDFs
3.3. Strain Sensing Functions of DCDFs
3.4. Cell Morphology, Proliferation, and Differentiation of NSCs on DCDFs
3.5. The Potential as Conductive Coating Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tsai, Y.-L.; Hsu, S.-h. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Azumi, R.; Shimada, S. A highly durable, stretchable, transparent and conductive carbon nanotube–polymeric acid hybrid film. Nanoscale 2019, 11, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.Y.; Shih, C.C.; Lee, W.Y.; Chueh, C.C.; Jang, G.W.; Huang, C.J.; Tung, S.H.; Chen, W.C. Bio-Based Transparent Conductive Film Consisting of Polyethylene Furanoate and Silver Nanowires for Flexible Optoelectronic Devices. Macromol. Rapid Commun. 2018, 39, 1800271. [Google Scholar] [CrossRef]
- Black, B.J.; Ecker, M.; Stiller, A.; Rihani, R.; Danda, V.R.; Reed, I.; Voit, W.E.; Pancrazio, J.J. In vitro compatibility testing of thiol-ene/acrylate-based shape memory polymers for use in implantable neural interfaces. J. Biomed. Mater. Res. Part A 2018, 106, 2891–2898. [Google Scholar] [CrossRef]
- Wu, J.-G.; Chen, J.-H.; Liu, K.-T.; Luo, S.-C. Engineering antifouling conducting polymers for modern biomedical applications. ACS Appl. Mater. Interfaces 2019, 11, 21294–21307. [Google Scholar] [CrossRef]
- Zhou, B.; Su, M.; Yang, D.; Han, G.; Feng, Y.; Wang, B.; Ma, J.; Ma, J.; Liu, C.; Shen, C. Flexible MXene/Silver Nanowire-Based Transparent Conductive Film with Electromagnetic Interference Shielding and Electro-Photo-Thermal Performance. ACS Appl. Mater. Interfaces 2020, 12, 40859–40869. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Chen, W.-C. Improved cell adhesion by plasma-induced grafting of l-lactide onto polyurethane surface. Biomaterials 2000, 21, 359–367. [Google Scholar] [CrossRef]
- Xu, J.; McCarthy, S.P.; Gross, R.A.; Kaplan, D.L. Chitosan Film Acylation and Effects on Biodegradability. Macromolecules 1996, 29, 3436–3440. [Google Scholar] [CrossRef]
- Joshi, M.; Adak, B.; Butola, B. Polyurethane nanocomposite based gas barrier films, membranes and coatings: A review on synthesis, characterization and potential applications. Prog. Mater. Sci. 2018, 97, 230–282. [Google Scholar] [CrossRef]
- Yang, H.; Yu, B.; Song, P.; Maluk, C.; Wang, H. Surface-coating engineering for flame retardant flexible polyurethane foams: A critical review. Compos. Part B Eng. 2019, 176, 107185. [Google Scholar] [CrossRef]
- Thangavelu, S.A.G.; Murali, A.; Sharanya, M.; Jaisankar, S.N.; Mandal, A.B. Studies on biodegradable polyurethane-SWCNTs nanocomposite films by covalent approach: Physicochemical, electric and mechanical properties. Appl. Surf. Sci. 2018, 449, 745–754. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Sheng, A.; Duan, H.; Zhao, G.; Liu, Y. Layered structural design of flexible waterborne polyurethane conductive film for excellent electromagnetic interference shielding and low microwave reflectivity. Appl. Surf. Sci. 2019, 469, 1–9. [Google Scholar] [CrossRef]
- Lee, T.-H.; Yen, C.-T.; Hsu, S.-H. Preparation of polyurethane-graphene nanocomposite and evaluation of neurovascular regeneration. ACS Biomater. Sci. Eng. 2020, 6, 597–609. [Google Scholar] [CrossRef]
- Ou, C.-W.; Su, C.-H.; Jeng, U.-S.; Hsu, S.-h. Characterization of biodegradable polyurethane nanoparticles and thermally induced self-assembly in water dispersion. ACS Appl. Mater. Interfaces 2014, 6, 5685–5694. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsu, S.-H. Synthesis and characterization of dual stimuli-sensitive biodegradable polyurethane soft hydrogels for 3D cell-laden bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 29273–29287. [Google Scholar] [CrossRef]
- Lin, T.-W.; Hsu, S.-H. Self-Healing Hydrogels and Cryogels from Biodegradable Polyurethane Nanoparticle Crosslinked Chitosan. Adv. Sci. 2020, 7, 1901388. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Hsu, S.-h. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Tao, L.; Hsieh, F.-Y.; Wei, Y.; Chiu, I.-M.; Hsu, S.-h. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Hoque, N.A.; Biswas, P.; Pal, K.; Thakur, P.; Das, K.; Karmakar, P. Antimicrobial and biocompatible fluorescent hydroxyapatite-chitosan nanocomposite films for biomedical applications. Colloids Surf. B Biointerfaces 2018, 171, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qin, J.; Zhang, P.; Chen, X.; You, X.; Zhang, F.; Zuo, B.; Yao, M. Facile preparation of a strong chitosan-silk biocomposite film. Carbohydr. Polym. 2020, 229, 115515. [Google Scholar] [CrossRef]
- Kenry; Liu, B. Recent advances in biodegradable conducting polymers and their biomedical applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef]
- Xu, J.; Wong, C.-W.; Hsu, S.-H. An Injectable, Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing. Chem. Mater. 2020, 32, 10407–10422. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chuang, W.-T.; Hsu, S.-H. Gelation mechanism and structural dynamics of chitosan self-healing hydrogels by in situ SAXS and coherent X-ray scattering. ACS Macro Lett. 2019, 8, 1449–1455. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-Inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Patricio, T.; Bártolo, P. Thermal stability of PCL/PLA blends produced by physical blending process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Sun, B.; Wu, T.; Wang, J.; Li, D.; Wang, J.; Gao, Q.; Bhutto, M.A.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous membranes promoting neural cell proliferation and differentiation with electrical stimulation. J. Mater. Chem. B 2016, 4, 6670–6679. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, H.-X.; Li, X.; Xu, W.-J.; Yin, Y.-x.; Dai, H.-l.; Wang, X.-b.; Huang, Z.-J.; Xu, P.-H. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. RSC Adv. 2018, 8, 10806–10817. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Cheng, B.; Bao, X.; Wang, Z.; Shangguan, Y.; Hu, Q. Self-healing and conductivity of chitosan-based hydrogels formed by the migration of ferric ions. J. Appl. Polym. Sci. 2019, 136, 47885. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Xie, H.G.; Zheng, J.N.; Li, X.X.; Liu, X.D.; Zhu, J.; Wang, F.; Xie, W.Y.; Ma, X.J. Effect of Surface Morphology and Charge on the Amount and Conformation of Fibrinogen Adsorbed onto Alginate/Chitosan Microcapsules. Langmuir 2010, 26, 5587–5594. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Fang, Y.; Wang, X. Maleic anhydride surface-modification of crosslinked chitosan membrane and its pervaporation performance. J. Membr. Sci. 2007, 295, 130–138. [Google Scholar] [CrossRef]
- Chen, T.W.; Chang, S.J.; Niu, G.C.-C.; Hsu, Y.T.; Kuo, S.M. Alginate-coated chitosan membrane for guided tissue regeneration. J. Appl. Polym. Sci. 2006, 102, 4528–4534. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, Y.-c.; Chuang, W.-T.; Jeng, U.-S.; Hsu, S.-H. Preparation, characterization, and mechanism for biodegradable and biocompatible polyurethane shape memory elastomers. ACS Appl. Mater. Interfaces 2017, 9, 5419–5429. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trung, T.Q.; Dang, T.M.L.; Ramasundaram, S.; Toi, P.T.; Park, S.Y.; Lee, N.-E. A Stretchable Strain-Insensitive Temperature Sensor Based on Free-Standing Elastomeric Composite Fibers for On-Body Monitoring of Skin Temperature. Acs Appl. Mater. Interfaces 2019, 11, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-h.; Lin, Y.; Lin, T.-C.; Tseng, T.-C.; Lee, H.-T.; Liao, Y.-C.; Chiu, I.-M. Spheroid formation from neural stem cells on chitosan membranes. J. Med. Biol. Eng. 2012, 32, 85–90. [Google Scholar] [CrossRef]
- Ren, Y.-J.; Zhang, H.; Huang, H.; Wang, X.-M.; Zhou, Z.-Y.; Cui, F.-Z.; An, Y.-H. In vitro behavior of neural stem cells in response to different chemical functional groups. Biomaterials 2009, 30, 1036–1044. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Gu, Y.; Lin, S.; Ding, F.; Gu, X. Effect of chitooligosaccharide on neuronal differentiation of PC-12 cells. Cell Biol. Int. 2009, 33, 352–356. [Google Scholar] [CrossRef]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.; Moulton, S.E.; Kapsa, R.M.I.; Wallace, G.G.; Crook, J.M. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng. Part C Methods 2015, 21, 385–393. [Google Scholar] [CrossRef]









| Abbreviation | DAPU/wt % | CEC/wt % | DCP/wt % | Conductivity/mS·cm−1 | Roughness/μm |
|---|---|---|---|---|---|
| Control | 50.00 | 50.00 | 0 | 0.11 ± 0.01 | 7.94 ± 0.54 |
| DCDF1 | 45.21 | 54.25 | 0.54 | 2.64 ± 0.18 | 8.05 ± 0.36 |
| DCDF2 | 49.75 | 49.75 | 0.50 | 2.83 ± 0.17 | 7.94 ± 0.23 |
| DCDF3 | 56.90 | 42.67 | 0.43 | 2.54 ± 0.15 | 8.40 ± 0.28 |
| Samples | Melting Temperature (Tm)/°C | Enthalpy (ΔHm)/J·g−1 | Crystallinity (Xc) | ||||
|---|---|---|---|---|---|---|---|
| PCL | CEC | PCL | CEC | PCL | CEC | ||
| Control | 46.47 | 139.53 | 151.98 | 11.03 | 10.13 | 22.67% | 10.71% |
| DCDF1 | 47.72 | 142.65 | 151.07 | 3.26 | 8.32 | 6.70% | 8.80% |
| DCDF2 | 47.67 | 144.00 | 155.08 | 8.86 | 8.02 | 18.21% | 8.48% |
| DCDF3 | 47.90 | 141.25 | 147.76 | 8.03 | 7.44 | 16.51% | 7.87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Fu, C.-Y.; Tsai, Y.-L.; Wong, C.-W.; Hsu, S.-h. Thermoresponsive and Conductive Chitosan-Polyurethane Biocompatible Thin Films with Potential Coating Application. Polymers 2021, 13, 326. https://doi.org/10.3390/polym13030326
Xu J, Fu C-Y, Tsai Y-L, Wong C-W, Hsu S-h. Thermoresponsive and Conductive Chitosan-Polyurethane Biocompatible Thin Films with Potential Coating Application. Polymers. 2021; 13(3):326. https://doi.org/10.3390/polym13030326
Chicago/Turabian StyleXu, Junpeng, Chih-Yu Fu, Yu-Liang Tsai, Chui-Wei Wong, and Shan-hui Hsu. 2021. "Thermoresponsive and Conductive Chitosan-Polyurethane Biocompatible Thin Films with Potential Coating Application" Polymers 13, no. 3: 326. https://doi.org/10.3390/polym13030326
APA StyleXu, J., Fu, C. -Y., Tsai, Y. -L., Wong, C. -W., & Hsu, S. -h. (2021). Thermoresponsive and Conductive Chitosan-Polyurethane Biocompatible Thin Films with Potential Coating Application. Polymers, 13(3), 326. https://doi.org/10.3390/polym13030326