Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
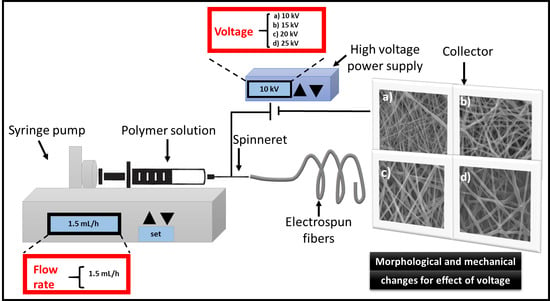
Preparation of the PCL Electrospun
2.2. Morphological Characterization of the PCL Electrospun Scaffolds
2.2.1. Scanning Electron Microscopy
2.2.2. Atomic Force Microscopy
2.2.3. Surface Area and Total Pore Volume by BET Method
2.3. Physicochemical Characterization of the PCL Scaffolds
2.3.1. Fourier-Transform Infrared Spectroscopy in Attenuated Total Reflectance (FTIR-ATR) Analysis
2.3.2. X-ray Diffraction (XRD) Analysis
2.3.3. Contact Angle Analysis
2.4. Mechanical Characterization of the Electrospun PCL Scaffolds
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphology of the Electrospun PCL Scaffolds
3.1.1. Scanning Electron Microscopy
3.1.2. Atomic Force Microscopy Analysis
3.1.3. Analysis of the Surface Area and Pore Volume by BET Method
3.2. Physicochemical Characterization of the Electrospun PCL Scaffolds
3.2.1. Fourier-Transform Infrared Spectroscopy in Attenuated Total Reflectance Mode
3.2.2. X-ray Diffraction
3.2.3. Contact Angle Measurements
3.3. Mechanical Characterization of PCL Electrospun Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Gough, C.R.; Deng, Q.; Gu, Z.; Wang, F.; Hu, X. Recent advanced in electrospun sustainable composites for medical, environmental, energy, and packaging applications. Int. J. Mol. Sci. 2020, 21, 4019. [Google Scholar] [CrossRef]
- Jiang, S.; Han, D.; Huang, C.; Duan, G.; Hou, H. Temperature-induced molecular orientation and mechanical properties of single electrospun polyimide nanofibers. Mater. Lett. 2018, 216, 81–83. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potential and perspective for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Fang, J.; Lin, T. Energy Harvesting Properties of Electrospun Nanofibers: Electrospinning: An Advanced Nanofiber Production Technology, 1st ed.; Niu, H., Zhou, H., Wang, H., Eds.; IOP Publishing Ltd.: Bristol, UK, 2020; pp. 1–44. [Google Scholar]
- Riaz, T.; Delaite, C.; Khenoussi, N.; Adolphe, D.; Schacher, L. A study of electrospinning and characterization of poly(caprolactone) nanofibers. J. Fashion Technol. Textile Eng. 2018, S5, 005. [Google Scholar] [CrossRef]
- Doustgani, A. Effect of electrospinning process parameters of polycaprolactone and nanohydroxyapatite nanocomposite nanofibers. Text. Res. J. 2015, 85, 1445–1454. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljevic, G.T.; Georgiadou, S. Porous electrospun polycaprolactone fibers: Effect of process parameters. J. Polym. Sci. Pol. Phys. 2016, 54, 1878–1888. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.I.; Sultana, N. Characterization, drug loading and antibacterial activity of nanohydroxyapatite/polycaprolactone (nHA/PCL) electrospun membrane. 3 Biotech 2017, 7, 249. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Duwez, A.-S.; Jérôme, C.; Léonard, A.F.; van der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Doustgani, A.; Vasheghani-Farahani, E.; Soleimani, M.; Hashemi-Najafabadi, S. Optimizing the mechanical properties of electrospun polycaprolactone and nanohydroxyapatite composites nanofibers. Compos. Part. B Eng. 2012, 43, 1830–1836. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldino, L.; Cardea, S.; Reverchon, E. A supercritical CO2 assisted electrohydrodynamic process used to produce microparticles and microfibers of a model polymer. J. CO2 Util. 2019, 33, 532–540. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Z.; Pan, Q.; Fang, T. Producing polymer fibers by electrospinning in supercritical fluids. J. Chem. 2013, 508905. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez, J.; Colchero, J.; Gomez, J.; Baro, A.A. A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Kundan, A.; Pradeep, K. Polycaprolactone composites with TiO2 for potential nanomaterials: Tunable properties using different phases. Phys. Chem. Chem. Phys. 2012, 14, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Nara, S.; Komiya, T. Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch. Starch/Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- ASTM-D882-12. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2012; Available online: www.astm.org (accessed on 22 February 2021). [CrossRef]
- Vanstrom, J.R. Mechanical Characterization of Commercial Biodegradable Plastic Films. Bachelor’s Thesis, Iowa State University, Ames, IA, USA, 2012. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Liu, Z.; Ju, K.; Wang, Z.; Li, W.; Ke, H.; He, J. Electrospun jets number and nanofiber morphology effected by voltage value: Numerical simulation and experimental verification. Nanoscale Res Lett. 2019, 14, 1. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Mazoochi, T.; Hamadanian, M.; Ahmadi, M.; Jabbari, V. Investigation on the morphological characteristics of nanofibrous membrane as electrospun in the different processing parameters. Int. J. Ind. Chem. 2012, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Dong, C.; Sheng, J. Morphology of ultrafine polysulfone fibers prepared by electrospinning. J. Polym. Int. 2004, 53, 1704–1710. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.; Ndesendo, V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 789289. [Google Scholar] [CrossRef] [Green Version]
- Kołbuk, D.; Sajkiewicz, P.; Kowalewski, T.A. Optical birefringence and molecular orientation of electrospun polycaprolactone fibers by polarizing-interference microscopy. Eur. Polym. J. 2012, 48, 275–283. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly (lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Sampson, W.W. Statistical geometry of pores and statistics of porous nanofibrous assemblies. J. R. Soc. Interface 2005, 2, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(e-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 10, 2796–2805. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Martins, A.; Pinho, E.; Faria, S.; Pashkuleva, I.; Marques, A.; Reis, R.; Neves, N. Surface modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological performance. Small 2009, 5, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Bolgen, N.; Salgado, A.J.; Gomes, E.; Piskin, E.; Reis, R.L. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005, 16, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and fiber fraction dominated wetting of electrospun fiber-based porous meshes. Polymers 2019, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Norton, D.; Ryan, A.J.; MacNeil, J.W. Investigation of fibroblast and keratinocyte cell-scaffold interactions using a novel 3D cell culture system. J. Mater. Sci. Mater. Med. 2007, 18, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwa, M.R.; Schwartz, A.G.; Xia, Y. Electrospun nanofibers for neural tissue engineering. Nanoscale 2010, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Telemeco, T.A.; Ayres, C.; Bowlin, G.L.; Wnek, G.E.; Boland, E.D.; Cohen, N.; Baumgarten, C.M.; Mathews, J.; Simpson, D.J. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Wang, S.; Ramakrishna, S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J. Biomed. Mater. Res. A 2004, 71, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, T.; Chew, S.Y. The application of nanofibrous scaffolds in neural tissue engineering. Adv. Drug. Deliv. Rev. 2009, 61, 1055–1064. [Google Scholar] [CrossRef]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Vozzi, G.; D’Acunto, M.; Brinzi, S.; Domenici, C.; Vozzi, F.; Ahluwalia, A.; Barbani, N.; Giusti, P.; Ciardelli, G. Characterization of blends between poly(e-caprolactone) and polysaccharides for tissue engineering applications. Mater. Sci. Eng. 2009, 7, 2174–2187. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Turng, L.S.; Li, Q. Crystalline morphology of electrospun poly(ε-caprolactone) (PCL) nanofibers. Ind. Eng. Chem. Res. 2013, 52, 4939–4949. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and characterization of poly(ε-caprolactone) nonwoven mats via melt electrospinning. Polymer 2012, 53, 248–253. [Google Scholar] [CrossRef]
- Thomas, V.; Moncy, V.; Chowdhury, S.; Sullivan, J.F.; Dean, D.R.; Vohra, Y.K. Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J. Biomater. Sci.-Polym. Ed. 2006, 9, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Küçükgöksel, Y.; Cesur, S. The investigation of desired product properties of polycaprolactone-hydroxy apatite composites for tissue engineering applications. Usak Univ. J. Mater. Sci. 2014, 1, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Chun, I. Nanometre diameter fibers of polymer produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.; Bowlin, G.L. Electrospinning jets and nanofibrous structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.H.; Lee, B.T. Fabrication and characterization of cross-linked gelatin. J. Biomed. Sci. Eng. 2010, 3, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.F.; Xia, Z.; Wong, S.-C.; Baji, A. Modelling of mechanical properties of electrospun nanofiber network. Int. J. Exp. Comput. Biomech. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- You, Y.; Lee, S.W.; Lee, S.J.; Park, W.H. Thermal interfiber bonding of electrospun poly (L-lactic acid) nanofibers. Mater. Lett. 2006, 60, 1331–1333. [Google Scholar] [CrossRef]
- Karuppuswamy, P.; Reddy, J. Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Bashur, C.A.; Goldstein, A.S.; Guelcher, S.A.; Barocas, V.H. Computational predictions of the tensile properties of electrospun fiber meshes: Effect of fiber diameter and fiber orientation. J. Mech. Behav. Biomed. Mater. 2008, 1, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Pawlowski, K.J.; Bowlin, G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly (glycolic acid) electrospinning. J. Macromol. Sci. 2001, 38, 1231–1243. [Google Scholar] [CrossRef]
- Boland, E.D.; Coleman, B.D.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Berhan, L.; Sastry, A.M. On modeling bonds in fused, porous networks: 3D Simulations of fibrous particulate joints. J. Compos. Mater. 2003, 37, 715–740. [Google Scholar] [CrossRef]









| Parameters | Voltage (kV) | |||
|---|---|---|---|---|
| 10 | 15 | 20 | 25 | |
| Crystallinity Index (%) | 47 | 47 | 48 | 47 |
| Contact angle (°) | 125 ± 1 * | 127 ± 2 | 130 ± 2 | 131 ± 1 * |
| Glass Transition Temp (°C) | −62 | −61 | −60 | −60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13, 662. https://doi.org/10.3390/polym13040662
Can-Herrera LA, Oliva AI, Dzul-Cervantes MAA, Pacheco-Salazar OF, Cervantes-Uc JM. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers. 2021; 13(4):662. https://doi.org/10.3390/polym13040662
Chicago/Turabian StyleCan-Herrera, L.A., A.I. Oliva, M.A.A. Dzul-Cervantes, O.F. Pacheco-Salazar, and J.M. Cervantes-Uc. 2021. "Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage" Polymers 13, no. 4: 662. https://doi.org/10.3390/polym13040662
APA StyleCan-Herrera, L. A., Oliva, A. I., Dzul-Cervantes, M. A. A., Pacheco-Salazar, O. F., & Cervantes-Uc, J. M. (2021). Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers, 13(4), 662. https://doi.org/10.3390/polym13040662