Polypropylene/Ethylene—And Polar—Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability
Abstract
:1. Introduction
2. Materials and Methods
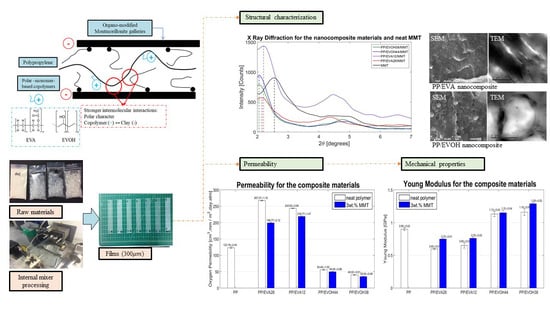
2.1. Dispersion and Morphologies of the Composites
2.2. Degree of Cristallinity of Polymers
2.3. Barrier Properties of the Nanocomposites and Polymer Blend
2.4. Mechanical Properties of the Nanocomposites and Polymer Blends
3. Results and Discussion
3.1. Polymeric Blends and Nanocomposites Morphologies and Structures
3.2. Oxygen Permeability and Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smithers, P. Sustainability and Its Impact on the Packaging Market (2020–2025). In Proceedings of the ACI´s 2nd European Food & Beverage Plastic Packaging Summit, Berlin, Germany, 8–9 February 2017. [Google Scholar]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging? Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Choudalakis, G.; Gotsis, A. Free volume and mass transport in polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 132–140. [Google Scholar] [CrossRef]
- Ratnakar, R. Structure and Properties of Nanoclay Reinforced Polymer Films, Fibers and Nonwovens. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2009. [Google Scholar]
- Vaia, R.A.; Giannelis, E.P. Polymer Melt Intercalation in Organically-Modified Layered Silicates: Model Predictions and Experiment. Macromolecules 1997, 30, 8000–8009. [Google Scholar] [CrossRef]
- Tjong, S. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Kim, H.; Fasulo, P.D.; Rodgers, W.R.; Paul, D.R. Structure and properties of polypropylene-based nanocomposites: Effect of PP-g-MA to organoclay ratio. Polymer 2007, 48, 5308–5323. [Google Scholar] [CrossRef]
- Mittal, V. Gas permeation and mechanical properties of polypropylene nanocomposites with thermally-stable imidazolium modified clay. Eur. Polym. J. 2007, 43, 3727–3736. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Khayet, M.; Manchado, M.A.L.; Valentin, J.L.; Seoane, B.; Mengual, J. Gas transport properties of polypropylene/clay composite membranes. Eur. Polym. J. 2007, 43, 1132–1143. [Google Scholar] [CrossRef]
- Ebadi-Dehaghani, H.; Barikani, M.; Khonakdar, H.A.; Jafari, S.H.; Wagenknecht, U.; Heinrich, G. On O2 gas permeability of PP/PLA/clay nanocomposites: A molecular dynamic simulation approach. Polym. Test. 2015, 45, 139–151. [Google Scholar] [CrossRef]
- Vaia, R.A.; Giannelis, E.P. Lattice Model of Polymer Melt Intercalation in Organically-Modified Layered Silicates. Macromolecules 1997, 30, 7990–7999. [Google Scholar] [CrossRef]
- Gutierrez, G.; Medina, J. Análisis de Las Propiedades de Barrera de Polipropileno Nanoreforzado y Su Relación Con Su Morfología y Estructura Cristalina. Master’s Thesis, Universidad de los Andes, Bogotá, DC, USA, 2006. [Google Scholar]
- Kermagoret, A.; Debuigne, A.; Jérôme, C.; Detrembleur, C. Precision design of ethylene- and polar-monomer-based copolymers by organometallic-mediated radical polymerization. Nat. Chem. 2014, 6, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, D.; Maiti, S.N. Effect of flexibility of ethylene vinyl acetate and crystallization of polypropylene on the mechanical properties of i-PP/EVA blends. J. Appl. Polym. Sci. 2011, 123, 1905–1912. [Google Scholar] [CrossRef]
- Ramírez-Vargas, E.; Navarro-Rodríguez, D.; Huerta-Martínez, B.M.; Medellín-Rodríguez, F.J.; Lin, J.S. Morphological and mechanical properties of polypropylene [PP]/poly(ethylene vinyl acetate) [EVA] blends. I. Homopolymer PP/EVA systems. Polym. Eng. Sci. 2000, 40, 2241–2250. [Google Scholar] [CrossRef]
- Ramírez-Vargas, E.; Medellín-Rodríguez, F.J.; Navarro-Rodríguez, D.; Avila-Orta, C.A.; Solís-Rosales, S.G.; Lin, J.S. Morphological and mechanical properties of polypropylene [PP]/poly(ethylene vinyl acetate) [EVA] blends. II: Polypropylene-(ethylene-propylene) heterophasic copolymer [PP-EP]/EVA systems. Polym. Eng. Sci. 2002, 42, 1350–1358. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef] [Green Version]
- Ares, A.; Silva, J.; Maia, J.M.; Barral, L.; Abad, M.-J.; Ares-Pernas, A. Rheomechanical and morphological study of compatibilized PP/EVOH blends. Rheol. Acta 2009, 48, 993–1004. [Google Scholar] [CrossRef]
- Ait-Kadi, A.; Bousmina, M.; Yousefi, A.; Mighri, F. High performance structured polymer barrier films obtained from compatibilized polypropylene/ethylene vinyl alcohol blends. Polym. Eng. Sci. 2007, 47, 1114–1121. [Google Scholar] [CrossRef]
- Son, T.W.; Lim, S.K.; Lee, D.W.; Lee, E.W. Physical Modification of Polypropylene. III. Novel Morphology of Polypro-pylene and Poly(ethylene-co-vinyl alcohol) with Epoxy Blend Fibers. J. Appl. Polym. Sci. 1999, 1049–1067. [Google Scholar] [CrossRef]
- Abad, M.-J.; Ares, A.; Barral, L.; Cano, J.; Díez, F.J.; García-Garabal, S.; López, J.; Ramírez, C. Use of a sodium ionomer as a compatibilizer in polypropylene/high-barrier ethylene-vinyl alcohol copolymer blends: The processability of the blends and their physical properties. J. Appl. Polym. Sci. 2004, 94, 1763–1770. [Google Scholar] [CrossRef]
- Özen, I.; Menceloglu, Y.Z. Barrier Properties of Polypropylene/Poly(M-Xylene Adipamide) and Polypropyl-ene/Poly(Ethylene-co-Vinyl Alcohol) Blend Films. J. Plast. Film Sheeting 2010, 377–394. [Google Scholar] [CrossRef]
- Li, T.; Dai, Y.; Li, J.; Guo, S.; Xie, G. A high-barrier PP/EVOH membrane prepared through the multistage biaxial-stretching extrusion. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Beltrán, M.; Benavente, V.; Marchante, V.; Marcilla, A. The influence of surfactant loading level in a montmorillonite on the thermal, mechanical and rheological properties of EVA nanocomposites. Appl. Clay Sci. 2013, 83, 153–161. [Google Scholar] [CrossRef]
- Su, Y.Y.; Rwei, S.P.; Gou, W.J.; Chan, H.H.; Cheng, K.C. Effect of polar interactions on the structure and rheology of EVA/Montmorillonite nanocomposites. J. Thermoplast. Compos. Mater. 2011, 25, 987–1003. [Google Scholar] [CrossRef]
- Ran, Q.; Hua, H.; Tian, Y.; Wu, S.; Shen, J. Preparation and Characterization of EVA/MMT Nanocomposites. Polym. Polym. Compos. 2006, 14, 301–306. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Wang, J.; Zong, R.; Gui, Z.; Chen, Z.; Zhuang, Y.; Fan, W. Influence of organophilic clay and preparation methods on EVA/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2003, 91, 2416–2421. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, K.R.; Han, S.-H.; Jeong, Y.S.; Kim, M.-N.; Park, E.-S. EVOH-based nanocomposites prepared by simple saponification method. J. Reinf. Plast. Compos. 2011, 30, 932–944. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Guo, M.; Zhang, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Ethylene-vinyl Alcohol Copolymer–Montmorillonite Multilayer Barrier Film Coated with Mulberry Anthocyanin for Freshness Monitoring. J. Agric. Food Chem. 2018, 66, 13268–13276. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.; Perez, J.G.; Sánchez-Soto, M.; Santana, O.O.; Maspoch, M.L. The effect of organo-modifier on the structure and properties of poly[ethylene-(vinyl alcohol)]/organo-modified montmorillonite composites. Polym. Int. 2010, 59, 778–786. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Marega, C.; Marigo, A.; Lorenzetti, C. Synergistic effect of dipentaerythritol and montmorillonite in EVOH-based nanocomposites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Goodarzi, V.; Jafari, S.H.; Khonakdar, H.A.; Seyfi, J. Morphology, rheology and dynamic mechanical properties of PP/EVA/clay nanocomposites. J. Polym. Res. 2011, 18, 1829–1839. [Google Scholar] [CrossRef]
- Mata-Padilla, J.M.; Medellín-Rodríguez, F.J.; Ávila-Orta, C.A.; Ramírez-Vargas, E.; Cadenas-Pliego, G.; Valera-Zaragoza, M.; Vega-Díaz, S.M. Morphology and chain mobility of reactive blend nanocomposites of PP-EVA/Clay. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Martins, C.; LaRocca, N.; Paul, D.; Pessan, L. Nanocomposites formed from polypropylene/EVA blends. Polymer 2009, 50, 1743–1754. [Google Scholar] [CrossRef]
- Olivares-Maldonado, Y.; Ramírez-Vargas, E.; Sánchez-Valdés, S.; Ramos-Devalle, L.F.; Rodriguez-Fernandez, O.S.; Espinoza-Martínez, A.B.; Medellín-Rodríguez, F.J.; Lozano-Ramirez, T. Effect of organoclay structure characteristics on properties of ternary PP-EP/EVA/nanoclay blend systems. Polym. Compos. 2014, 35, 2241–2250. [Google Scholar] [CrossRef]
- Kim, S.W.; Cha, S.-H. Thermal, mechanical, and gas barrier properties of ethylene-vinyl alcohol copolymer-based nanocomposites for food packaging films: Effects of nanoclay loading. J. Appl. Polym. Sci. 2014, 131, 1280–1288. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Adelnia, H.; Pour, R.H.; Soheilmoghaddam, M. Preparation and characterization of ethylene-vinyl acetate/halloysite nanotube nanocomposites. J. Mater. Sci. 2015, 50, 3237–3245. [Google Scholar] [CrossRef]
- Fried, J. Polymer Additives, Blends, and Composites. In Polymer Science and Technology; Fried, J., Ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1995; pp. 251–284. [Google Scholar]
- Groeninckx, G.; Vanneste, M.; Everaert, V. Crystallization, Morphological Structure, and Melting of Polymer Blends. In Polymer Blends Handbook; Utracki, L.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 203–289. [Google Scholar]
- Inoue, T. Morphology of Polymer Blends. In Polymer Blends Handbook; Utracki, L.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 547–574. [Google Scholar]
- Kim, J.S.; Jang, J.H.; Kim, J.H.; Lim, D.Y.; Lee, Y.S.; Chang, Y.-W.; Kim, D.H. Morphological, thermal, rheological, and mechanical properties of PP/EVOH blends compatibilized with PP-g-IA. Polym. Eng. Sci. 2016, 56, 1240–1247. [Google Scholar] [CrossRef]
- Luo, D.-J.; Shao, H.-J.; Wei, F.-J.; Zhang, K.-Z.; Cui, Z.-Y.; Yu, J.; Qin, S.-H. Morphology and Isothermal Crystallization Kinetics of Polypropylene/Poly(ethylene-co-vinyl alcohol) Blends. Int. Polym. Process. 2019, 34, 195–208. [Google Scholar] [CrossRef]
- Mittal, V. Crystallinity, mechanical property and oxygen permeability of polypropylene: Effect of processing conditions, nucleating agent and compatibilizer. J. Thermoplast. Compos. Mater. 2012, 1407–1423. [Google Scholar] [CrossRef]
- Mittal, V. Compatibilization of Interfaces in Nanocomposites: Route towards Better Barrier Properties. In Barrier Properties of Polymer Clay Nanocomposites; Mittal, V., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 19–41. [Google Scholar]
- Dogu, S.; Taky, E.; Sen, S. Effect of EVA-g-MA and EVACO compatibilizers/tougheners on morphological and mechanical properties of PP/EVA/HNT blend polymer nanocomposites. J. Compos. Mater. 2019, 1–21. [Google Scholar] [CrossRef]
- Varghese, A.M.; Rangaraj, V.M.; Mun, S.C.; Macosko, C.W.; Mittal, V. Effect of Graphene on Polypropylene/Maleic Anhydride-graft-Ethylene–Vinyl Acetate (PP/EVA-g-MA) Blend: Mechanical, Thermal, Morphological, and Rheological Properties. Ind. Eng. Chem. Res. 2018, 57, 7834–7845. [Google Scholar] [CrossRef]
- Joubert, C.; Cassagnau, P.; Michel, A.; Choplin, L. Influence of the processing conditions on a two-phase reactive blend system: EVA/PP thermoplastic vulcanizate. Polym. Eng. Sci. 2002, 42, 2222–2233. [Google Scholar] [CrossRef]
- Demarquette, N.R.; Kamal, M.R. Influence on Maleation of Polypropylene on the Interfacial Properties between Poly-propylene and Ethylene-Vinyl Alcohol Copolymer. J. Appl. Polym. Sci. 1998, 70, 75–87. [Google Scholar] [CrossRef]
- Subramanian, P.M.; Plotzker, I.G. Barrier Materials by Blending. In Polymer Blends. Volume 2: Performance; Paul, D.R., Bucknall, C.B., Eds.; John Wiley & Sons: New York, NY, USA, 2000; pp. 359–395. [Google Scholar]
- Faisant, J.; Aït-Kadi, A.; Bousmina, M.; Deschenes, L. Morphology, thermomechanical and barrier properties of polypropylene-ethylene vinyl alcohol blends. Polymer 1998, 39, 533–545. [Google Scholar] [CrossRef]
- Castel, C.D.; Bianchi, O.; Oviedo, M.; Liberman, S.; Mauler, R.; Oliveira, R. The influence of interfacial agents on the morphology and viscoelasticity of PP/MMT nanocomposites. Mater. Sci. Eng. C 2009, 29, 602–606. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the Barrier Properties of Polymer-Layered Silicate Nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Bhunia, K.; Dhawan, S.; Sablani, S.S. Modeling the Oxygen Diffusion of Nanocomposite-based Food Packaging Films. J. Food Sci. 2012, 77, N29–N38. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, J.; Ray, S.S.; Scriba, M.; Wesley-Smith, J. A combined experimental and theoretical approach to establish the relationship between shear force and clay platelet delamination in melt-processed polypropylene nanocomposites. Polymer 2014, 55, 2233–2245. [Google Scholar] [CrossRef]
- Mittal, V. Self-Consistent Field Theory Modeling of Polymer Nanocomposites. In Modeling and Prediction of Polymer Nanocomposite Properties; Mittal, V., Ed.; Wiley: Weinheim, Germany, 2013; pp. 11–37. [Google Scholar]
- Vijayan, P.; Varghese, S.; Thomas, S. Mechanical and Viscoelastic Characterization of Multiphase Polymer Systems. In Handbook of Multiphase Polymer Systems; Boudenne, A., Ibos, L., Candau, Y., Thomas, S., Eds.; John Wiley & Sons: Chichester, UK, 2011; pp. 251–311. [Google Scholar]




| Materials | Sample Name | Composition [wt %] | |||
|---|---|---|---|---|---|
| PP | EVA | EVOH | MMT | ||
| Neat polymers | PP | 100 | − | − | − |
| EVA12 | − | 100 | − | − | |
| EVA28 | − | 100 | − | − | |
| EVOH38 | − | − | 100 | − | |
| EVOH44 | − | − | 100 | − | |
| Neat Polymer blends | PP/EVA12 | 75.0 | 25.0 | − | 0 |
| PP/EVA28 | 75.0 | 25.0 | − | 0 | |
| PP/EVOH38 | 75.0 | − | 25.0 | 0 | |
| PP/EVOH44 | 75.0 | − | 25.0 | 0 | |
| Nanocomposites | PP/EVA12/MMT | 72.8 | 24.2 | − | 3.0 |
| PP/EVA28/MMT | 72.8 | 24.2 | − | 3.0 | |
| PP/EVOH38/MMT | 72.8 | − | 24.2 | 3.0 | |
| PP/EVOH44/MMT | 72.8 | − | 24.2 | 3.0 | |
| Type of Material | Material | Tm [°C] | Tc [°C] | Degree of Crystallinity [%] | |||
|---|---|---|---|---|---|---|---|
| PP | Ethylene and Polar Monomer Copolymer | PP | Ethylene and Polar Monomer Copolymer | PP | Ethylene- and Polar- Monomer-Copolymer | ||
| Neat Polymers | PP | 142.91 | − | 102.98 | − | 34 ± 2 | − |
| EVA12 | − | 86.82 | − | 72.05 | − | 14 ± 1 | |
| EVA28 | − | 73.38 | − | 54.22 | − | 22 ± 1 | |
| EVOH38 | − | 162.55 | − | 142.71 | − | 37 ± 2 | |
| EVOH44 | − | 157.11 | − | 137.75 | − | 34 ± 2 | |
| Neat Polymer blends | PP/EVA12 | 143.08 | 87.37 | 105.84 | 71.47 | 17 ± 1 | 10 ± 1 |
| PP/EVA28 | 143.91 | 78.40 | 102.13 | 54.23 | 15 ± 1 | 3 ± 1 | |
| PP/EVOH38 | 147.97 | 164.55 | 114.85 | 143.40 | 38 ± 2 | 31 ± 1 | |
| PP/EVOH44 | 148.45 | 164.65 | 114.64 | 138.60 | 38 ± 1 | 28 ± 1 | |
| Nanocomposites | PP/EVA12/MMT | 144.71 | 87.19 | 107.24 | 71.57 | 15 ± 1 | 10 ± 1 |
| PP/EVA28/MMT | 145.34 | 77.88 | 105.42 | 56.24 | 16 ± 1 | 3 ± 1 | |
| PP/EVOH38/MMT | 148.54 | 159.18 | 115.47 | 142.70 | 28 ± 2 | 28 ± 1 | |
| PP/EVOH44/MMT | 148.01 | 159.56 | 115.30 | 140.40 | 34 ± 1 | 31 ± 1 | |
| Type of Material | Material | Young Modulus [GPa] | Elongation at Break [%] | Tensile Strength [MPa] |
|---|---|---|---|---|
| Neat Polymers | PP | 0.96 ± 0.04 | 690 ± 10 | 22.30 ± 4.00 |
| EVA12 | 0.10 ± 0.05 | 825 ± 60 | 14.88 ± 3.27 | |
| EVA28 | 0.09 ± 0.04 | 900 ± 50 | 16.24 ± 4.21 | |
| EVOH38 | 2.32 ± 0.21 | 43 ± 21 | 57.41 ± 3.12 | |
| EVOH44 | 1.83 ± 0.23 | 48 ± 18 | 45.05 ± 7.34 | |
| Polymeric blends | PP/EVA12 | 0.65 ± 0.04 | 587 ± 45 | 17.62 ± 0.64 |
| PP/EVA28 | 0.60 ± 0.01 | 310 ± 42 | 16.85 ± 0.63 | |
| PP/EVOH38 | 1.16 ± 0.05 | 6 ± 2 | 20.28 ± 2.51 | |
| PP/EVOH44 | 1.13 ± 0.04 | 16 ± 7 | 19.01 ± 1.89 | |
| Nanocomposites materials | PP/EVA12/MMT | 0.76 ± 0.02 | 574 ± 58 | 17.89 ± 0.61 |
| PP/EVA28/MMT | 0.75 ± 0.01 | 353 ± 55 | 17.42 ± 0.45 | |
| PP/EVOH38/MMT | 1.29 ± 0.02 | 13 ± 8 | 19.95 ± 1.05 | |
| PP/EVOH44/MMT | 1.15 ± 0.04 | 19 ± 10 | 17.94± 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Landinez, J.F.; Salcedo-Galan, F.; Medina-Perilla, J.A. Polypropylene/Ethylene—And Polar—Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability. Polymers 2021, 13, 705. https://doi.org/10.3390/polym13050705
Castro-Landinez JF, Salcedo-Galan F, Medina-Perilla JA. Polypropylene/Ethylene—And Polar—Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability. Polymers. 2021; 13(5):705. https://doi.org/10.3390/polym13050705
Chicago/Turabian StyleCastro-Landinez, Juan Felipe, Felipe Salcedo-Galan, and Jorge Alberto Medina-Perilla. 2021. "Polypropylene/Ethylene—And Polar—Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability" Polymers 13, no. 5: 705. https://doi.org/10.3390/polym13050705
APA StyleCastro-Landinez, J. F., Salcedo-Galan, F., & Medina-Perilla, J. A. (2021). Polypropylene/Ethylene—And Polar—Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability. Polymers, 13(5), 705. https://doi.org/10.3390/polym13050705