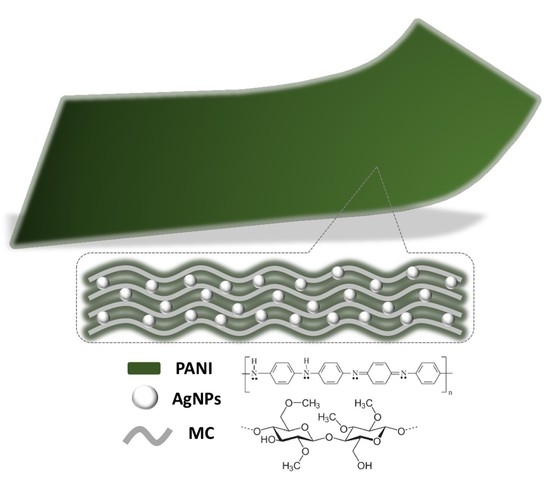
Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PANI
2.3. Molecular Weight (MW) Determination of PANI
2.4. Preparation of MC/PANI/AgNPs Composite Film
2.5. Characterization Techniques
3. Results and Discussion
3.1. Structural Investigation of the Synthesized Films
3.2. Optical Properties of the Synthesized Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Cheng, Q.; Jiang, B.; Huang, Y. Design of the novel polyaniline/polysiloxane flexible nanocomposite film and its application in gas sensor. Compos. Part B Eng. 2020, 196, 108131. [Google Scholar] [CrossRef]
- Lotfy, S.; Atta, A.; Abdeltwab, E. Comparative study of gamma and ion beam irradiation of polymeric nanocomposite on electrical conductivity. J. Appl. Polym. Sci. 2018, 135, 46146. [Google Scholar] [CrossRef]
- Shandilya, M.; Thakur, S.; Thakur, S. Magnetic amendment in the fabrication of environment friendly and biodegradable iron oxide/ethyl cellulose nanocomposite membrane via electrospinning. Cellulose 2020, 27, 10007–10017. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Q.; Wang, D.; Zeng, X.F.; Wang, J.X.; Chen, J.F. High-gravity-assisted synthesis of surfactant-free transparent dispersions of monodispersed MgAl-LDH nanoparticles. Ind. Eng. Chem. Res. 2020, 59, 2960–2967. [Google Scholar] [CrossRef]
- Das, M.; Sarker, A.K. Multilayer Engineering of Polyaniline and Reduced Graphene Oxide Thin Films on a Plastic Substrate for Flexible Optoelectronic Applications Using NIR. Russ. J. Appl. Chem. 2020, 93, 1561–1570. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, H.; Li, G.; Fu, B.; Bao, X.; Wang, Z.; Hu, Q. Stretchable, conductive PAni-PAAm-GOCS hydrogels with excellent mechanical strength, strain sensitivity and skin affinity. Chem. Eng. J. 2020, 394, 124901. [Google Scholar] [CrossRef]
- Liebeck, B.M.; Hidalgo, N.; Roth, G.; Popescu, C.; Böker, A. Synthesis and characterization of methyl cellulose/keratin hydrolysate composite membranes. Polymers 2017, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, Q.; Luo, G.; Meng, W.; Cao, M.; Li, Y.; Masterman-Smith, M.D.; Yang, H.; Sun, X.; Lang, M.-F. A novel flexible Ag/AgCl quasi-reference electrode based on silver nanowires toward ultracomfortable electrophysiology and sensitive electrochemical glucose detection. J. Mater. Res. Technol. 2020, 9, 13425–13433. [Google Scholar] [CrossRef]
- Lu, Z.; Yao, C.; Xie, F.; Si, L.; Jia, F.; Huang, J.; Ma, Q. Highly flexible and conductive sodium carboxymethyl cellulose/silver nanowires composite films. J. Mater. Sci. Mater. Electron. 2020, 31, 2353–2359. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, F.; Wen, K.; Lu, J. Enhanced photocatalytic activity for tetracyclines degradation with Ag modified g-C3N4 composite under visible light. J. Photochem. Photobiol. A Chem. 2020, 389, 112217. [Google Scholar] [CrossRef]
- Ullah, N.; Li, D.; Xiaodong, C.; Yasin, S.; Umair, M.M.; Eede, V.; Wei, Q. Photo–irradiation based biosynthesis of silver nanoparticles by using an ever green shrub and its antibacterial study. Dig. J. Nanomater. Biostruct. 2015, 10, 95–105. [Google Scholar]
- Ullah, N.; Yasin, S.; Abro, Z.; Liu, L.; Wei, Q. Mechanically robust and antimicrobial cotton fibers loaded with silver nanoparticles: Synthesized via Chinese holly plant leaves. Int. J. Text. Sci. 2014, 3, 1–5. [Google Scholar]
- Abdelhamied, M.; Atta, A.; Abdelreheem, A.; Farag, A.; El Okr, M. Synthesis and Optical Properties of PVA/PANI/Ag Nanocomposite films. J. Mater. Sci. Mater. Electron. 2020, 31, 22629–22641. [Google Scholar] [CrossRef]
- Pawcenis, D.; Syrek, M.; Aksamit-Koperska, M.A.; Łojewski, T.; Łojewska, J. Mark–Houwink–Sakurada coefficients determination for molar mass of silk fibroin from viscometric results. SEC-MALLS approach. RSC Adv. 2016, 6, 38071–38078. [Google Scholar] [CrossRef]
- Lu, Y.; Song, Y.; Wang, F. Thermoelectric properties of graphene nanosheets-modified polyaniline hybrid nanocomposites by an in situ chemical polymerization. Mater. Chem. Phys. 2013, 138, 238–244. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.S.; Jilani, A.; Yahia, I.S.; Al-Ghamdi, A.A. Enhanced the photocatalytic activity of Ni-doped ZnO thin films: Morphological, optical and XPS analysis. Superlattices Microstruct. 2016, 94, 108–118. [Google Scholar] [CrossRef]
- Hebeish, A.; Sharaf, S. Novel nanocomposite hydrogel for wound dressing and other medical applications. RSC Adv. 2015, 5, 103036–103046. [Google Scholar] [CrossRef]
- Gasaymeh, S.S.; ALmansoori, N.N. Novel formation mechanism of Ag/PANI/PVP core-shell nanocomposites. Results Phys. 2020, 16, 102882. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and structural analysis of polyaniline and poly (o-anisidine) layers generated in a DC glow discharge plasma by using an oblique angle electrode deposition configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.; Zafar, A. Biosynthesis and characterization of silver nanoparticles using Phoenix dactylifera fruits extract and their in vitro antimicrobial and cytotoxic effects. Open Biotechnol. J. 2019, 13, 37–46. [Google Scholar] [CrossRef]
- Gottam, R.; Srinivasan, P. Composite electrode material of MoO3-MC-SiO2-PANI: Aqueous supercapacitor cell with high energy density, 1 V and 250,000 CD cycles. Polym. Adv. Technol. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Liang, Y.; An, W.; Wang, H.; Liu, L.; Hu, J.; Cui, W. Construction of Z-scheme heterojunction of PANI-Ag-CN sandwich structure with efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 509, 145296. [Google Scholar] [CrossRef]
- Abdel-Galil, A.; Atta, A.; Balboul, M.R. Effect of low energy oxygen ion beam treatment on the structural and physical properties of ZnO thin films. Surf. Rev. Lett. 2020, 27, 20500195. [Google Scholar] [CrossRef]
- Chintala, K.M.; Panchal, S.; Rana, P.; Chauhan, R. Structural, optical and electrical propertie of gamma-rays exposed selenium nanowires. J. Mater. Sci. Mater. Electron. 2016, 27, 8087–8093. [Google Scholar] [CrossRef]
- Abdel-Galil, A.; Assiri, M.; Yahia, I. Optical analysis of methyl violet thin films/polymeric substrate for flexible organic technology. Opt. Quantum Electron. 2020, 52, 1–20. [Google Scholar] [CrossRef]
- Atta, A.; Abdel Reheem, A.M.; Abdeltwab, E. Ion beam Irradiation effects on surface morphology and optical properties of ZnO/PVA composites. Surf. Rev. Lett. 2020, 27, 1950214. [Google Scholar] [CrossRef]
- Soliman, T.; Abouhaswa, A. Synthesis and structural of Cd0. 5Zn0. 5F2O4 nanoparticles and its influence on the structure and optical properties of polyvinyl alcohol films. J. Mater. Sci. Mater. Electron. 2020, 31, 9666–9674. [Google Scholar]
- Wad, S.; El-Gamal, S.; El Sayed, A.M.; Abdel-Hady, E.E. Characterization, optical, and nanoscale free volume properties of Na-CMC/PAM/CNT nanocomposites. Polym. Adv. Technol. 2020, 31, 114–125. [Google Scholar]
- Shen, D.; Wu, Y.; Lo, M.F.; Lee, C.S. Charge transport properties of co-evaporated organic–inorganic thin film charge transfer complexes: Effects of intermolecular interactions. J. Mater. Chem. C 2020, 8, 16725–16729. [Google Scholar] [CrossRef]
- Ledinsky, M.; Schönfeldová, T.; Holovský, J.; Aydin, E.; Hájková, Z.; Landová, L.; Neyková, N.; Fejfar, A.; De Wolf, S. Temperature dependence of the urbach energy in lead iodide perovskites. J. Phys. Chem. Lett. 2019, 10, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Galil, A.; Balboul, M.R.; Atta, A.; Yahia, I.S.; Sharaf, A. Preparation, structural and optical characterization of nanocrystalline CdS thin film. Phys. B Condens. Matter 2014, 447, 35–41. [Google Scholar] [CrossRef]
- Zeyada, H.; El-Nahass, M.; El-Shabaan, M. Gamma-ray irradiation induced structural and optical constants changes of thermally evaporated neutral red thin films. J. Mater. Sci. 2012, 47, 493–502. [Google Scholar] [CrossRef]
- Abdel Reheem, A.M.; Atta, A.; Afify, T.A. Optical and electrical properties of argon ion beam irradiated PVA/Ag nanocomposites. Surf. Rev. Lett. 2017, 24, 1750038. [Google Scholar] [CrossRef]
- Taha, T.A.; Hendawy, N.; El-Rabaie, S.; Esmat, A.; El-Mansy, M.K. Effect of NiO NPs doping on the structure and optical properties of PVC polymer films. Polym. Bull. 2019, 76, 4769–4784. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Labis, J.P.; Al-Assiri, M.S. A novel Ag/PANI/ZnTiO3 ternary nanocomposite as a highly efficient visible-light-driven photocatalyst. Sep. Purif. Technol. 2021, 256, 117847. [Google Scholar] [CrossRef]
- Yahia, I.S.; Alfaify, S.; Jilani, A.; Abdel-wahab, M.S.; Al-Ghamdi, A.A.; Abutalib, M.M.; El-Naggar, A.M. Non-linear optics of nano-scale pentacene thin film. Appl. Phys. B 2016, 122, 1–5. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Kravets, V.G.; Marshall, O.P.; Nair, R.R.; Thackray, B.; Zhukov, A.; Leng, J.; Grigorenko, A.N. Engineering optical properties of a graphene oxide metamaterial assembled in microfluidic channels. Opt. Express 2015, 23, 1265–1275. [Google Scholar] [CrossRef] [Green Version]
- Barde, R.V.; Nemade, K.R.; Waghuley, S.A. Complex optical study of V2O5–P2O5–B2O3–Dy2O3 glass systems. J. Taibah Univ. Sci. 2016, 10, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Kumar, S.; Thangaraj, R. Optical properties of polyvinyl alcohol doped (Se80Te20) 100–x Ag x (0 ≤ x ≤ 4) composites. Phase Transit. 2014, 87, 206–222. [Google Scholar] [CrossRef]
- Elsaeedy, H.I.; Ali, H.E.; Algarni, H.; Yahia, I.S. Nonlinear behavior of the current–voltage characteristics for erbium-doped PVA polymeric composite films. Appl. Phys. A 2019, 125, 79. [Google Scholar] [CrossRef]
- Shahriari, E.; Yunus, W.; Saion, E. Effect of particle size on nonlinear refractive index of Au nanoparticle in PVA solution. Braz. J. Phys. 2010, 40, 256–260. [Google Scholar] [CrossRef]
- Donya, H.; Taha, T.; Alruwaili, A.; Tomsah, I.; Ibrahim, M. Micro-structure and optical spectroscopy of PVA/iron oxide polymer nanocomposites. J. Mater. Res. Technol. 2020, 9, 9189–9194. [Google Scholar] [CrossRef]
- Ghanipour, M.; Dorranian, D. Effect of Ag-nanoparticles doped in polyvinyl alcohol on the structural and optical properties of PVA films. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- El-Nahass, M.; Farag, A.; Abd-El-Salam, F. Effect of gamma irradiation on the optical properties of nano-crystalline InP thin films. Appl. Surf. Sci. 2009, 255, 9439–9443. [Google Scholar] [CrossRef]
- Ali, F.M.; Kershi, R.M.; Sayed, M.A.; AbouDeif, Y.M. Evaluation of structural and optical properties of Ce3+ ions doped (PVA/PVP) composite films for new organic semiconductors. Phys. B Condens. Matter 2018, 538, 160–166. [Google Scholar] [CrossRef]
- Alwan, T.J. Gamma irradiation effect on the optical properties and refractive index dispersion of dye doped polystyrene films. Turk. J. Phys. 2012, 36, 377–384. [Google Scholar]
- El Sayed, A.; El-Sayed, S.; Morsi, W.; Mahrous, S.; Hassen, A. Synthesis, characterization, optical, and dielectric properties of polyvinyl chloride/cadmium oxide nanocomposite films. Polym. Compos. 2014, 35, 1842–1851. [Google Scholar] [CrossRef]
- Saadeddin, I.; Pecquenard, B.; Manaud, J.-P.; Decourt, R.; Labrugère, C.; Buffeteau, T. Synthesis and characterization of single-and co-doped SnO2 thin films for optoelectronic applications. Appl. Surf. Sci. 2007, 253, 5240–5249. [Google Scholar] [CrossRef]
- Hassouni, M.H.; Mishjil, K.A.; Chiad, S.S.; Habubi, N.F. Effect of gamma irradiation on the optical properties of Mg doped CdO Thin films deposited by Spray Pyrolysis. Int. Lett. Chem. Phys. Astron. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.B.; Abdel-Kader, M. Effect of excess oxygen content within different nano-oxide additives on the structural and optical properties of PVA/PEG blend. Appl. Phys. A 2019, 125, 209. [Google Scholar] [CrossRef]
- Frumar, M.; Jedelský, J.; Frumarova, B.; Wagner, T.; Hrdlička, M. Optically and thermally induced changes of structure, linear and non-linear optical properties of chalcogenides thin films. J. Non-Cryst. Solids 2003, 326, 399–404. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-wahab, M.S.; Al-ghamdi, A.A.; Sadik Dahlan, A.; Yahia, I.S. Nonlinear optical parameters of nanocrystalline AZO thin film measured at different substrate temperatures. Phys. B Condens. Matter 2016, 481, 97–103. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, K.P.; Tripathi, S.K. Study of linear and non-linear optical responses of MoSe 2–PMMA nanocomposites. J. Mater. Sci. Mater. Electron. 2020, 31, 19974–19988. [Google Scholar] [CrossRef]
- Sen, S.K.; Al Mortuza, A.; Manir, M.S.; Pervez, M.; Hossain, S.M.A.I.; Alam, M.S.; Haque, M.A.S.; Matin, M.A.; Hakim, M.A.; Huda, A.-U. Structural and optical properties of sol-gel synthesized h-MoO3 nanorods treated by gamma radiation. Nano Express 2020, 1, 020026. [Google Scholar] [CrossRef]
- Awed, A.S.; Maksoud, M.A.; Atta, M.M.; Fahim, R.A. Nonlinear optical properties of irradiated 1, 2-dihydroxyanthraquinone thin films: Merged experimental and TD-DFT insights. J. Mater. Sci. Mater. Electron. 2019, 30, 7858–7865. [Google Scholar] [CrossRef]
- Sakhno, O.; Yezhov, P.; Hryn, V.; Rudenko, V.; Smirnova, T. Optical and nonlinear properties of photonic polymer nanocomposites and holographic gratings modified with noble metal nanoparticles. Polymers 2020, 12, 480. [Google Scholar] [CrossRef] [Green Version]
- Borah, D.J.; Mostako, A.T.T. Investigation on dispersion parameters of Molybdenum Oxide thin films via Wemple–DiDomenico (WDD) single oscillator model. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Ravi, S.; Sreedharan, R.; Raghi, K.R.; Kumar, T.M.; Naseema, K. Experimental and computational perspectives on linear and non-linear optical parameters of an orthorhombic crystal for optical limiting applications. Appl. Phys. A 2020, 126, 1–19. [Google Scholar] [CrossRef]









| The Samples | Absorption Edge (Ed) (eV) | Optical Band Gap (Eg) (eV) | Urbach Energy (Ee) (eV) | Carbon Cluster Number (N) |
|---|---|---|---|---|
| Pure MC | 5.42 | 5.76 | 0.51 | 36 |
| MC/1%PANI | 3.70 | 5.06 | 1.38 | 45 |
| MC/3%PANI | 3.14 | 5.01 | 1.52 | 47 |
| MC/3%PANI/0.5%Ag | 2.38 | 4.41 | 1.92 | 61 |
| MC/3%PANI/1%Ag | 1.93 | 4.27 | 2.02 | 65 |
| The Samples | Eo (eV) | Ed (eV) | ε∞ | εl | N/m* × 1038 Kg−1 m−3 | Wp × 1012 (S−1) |
|---|---|---|---|---|---|---|
| Pure MC | 4.1 | 0.43 | 1.10 | 1.11 | 0.40 | 0.116 |
| MC/1%PANI | 5.12 | 0.74 | 1.12 | 1.14 | 0.25 | 0.072 |
| MC/3%PANI | 4.80 | 0.87 | 1.18 | 1.18 | 0.20 | 0.058 |
| MC/3%PANI/0.5%AgNPs | 3.87 | 0.90 | 1.23 | 1.25 | 0.60 | 0.171 |
| MC/3%PANI/1%AgNPs | 4.40 | 0.90 | 1.19 | 1.28 | 0.50 | 0.150 |
| The Films | no | λo (nm) | So × 1012 (m−2) | τ × 10−14 (s) |
|---|---|---|---|---|
| Pure MC | 1.05 | 412 | 0.58 | 8.8 |
| MC/1%PANI | 1.07 | 244 | 2.38 | 6.6 |
| MC/3%PANI | 1.18 | 346 | 3.20 | 5.7 |
| MC/3%PANI/0.5%Ag | 1.11 | 477 | 1.02 | 1.4 |
| MC/3%PANI/1%Ag | 1.26 | 500 | 2.35 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.; Abdelhamied, M.M.; Abdelreheem, A.M.; Berber, M.R. Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties. Polymers 2021, 13, 1225. https://doi.org/10.3390/polym13081225
Atta A, Abdelhamied MM, Abdelreheem AM, Berber MR. Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties. Polymers. 2021; 13(8):1225. https://doi.org/10.3390/polym13081225
Chicago/Turabian StyleAtta, Ali, Mostufa M. Abdelhamied, Ahmed M. Abdelreheem, and Mohamed R. Berber. 2021. "Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties" Polymers 13, no. 8: 1225. https://doi.org/10.3390/polym13081225
APA StyleAtta, A., Abdelhamied, M. M., Abdelreheem, A. M., & Berber, M. R. (2021). Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties. Polymers, 13(8), 1225. https://doi.org/10.3390/polym13081225