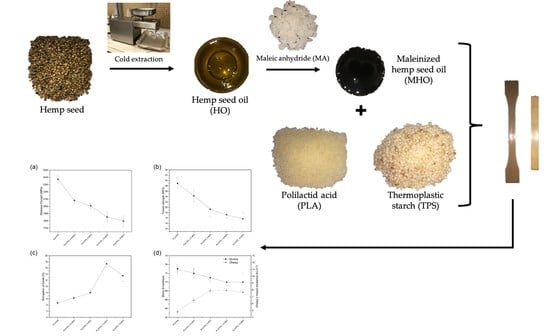
Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Maleinization of Hemp Seed Oil
2.3. Processing and Compatibilization of PLA/TPS
2.4. Mechanical and Thermal Characterization
2.5. Morphological Characterization
2.6. Disintegration under Composting Conditions
3. Results and Discussion
3.1. Synthesis of Maleinized Hemp Seed Oil
3.2. Mechanical Properties of PLA/TPS Blends with MHO
3.3. Morphological Characterization
3.4. Thermal Characterization
3.5. Disintegration under Composting Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATBC | acetyl tributyl citrate |
| C | the exact concentration of KOH |
| ΔHc | cold crystallization enthalpy |
| ΔHcc | crystallization enthalpy |
| ΔHm | melting enthalpy |
| melting enthalpy for a PLA structure theoretically fully crystalline | |
| DMTA | dynamic mechanical thermal analysis |
| DSC | differential scanning calorimetry |
| DTG | first derivate curves |
| EJO | epoxidized jatropha oil |
| EKO | epoxidized karanja oil |
| ELO | epoxidized linseed oil |
| EPO | epoxidized palm oil |
| ESO | epoxidized soybean oil |
| FESEM | field emission scanning electron microscope |
| G′ | storage modulus |
| HO | hemp seed oil |
| m | the mass of maleinized oil used to titrate it |
| MA | malic anhydride |
| MCSO | maleinized cottonseed oil |
| MHO | maleinized hempseed oil |
| MLO | maleinized linseed oil |
| OLA | oligomeric lactic acid |
| PBAT | polybutylene adipate-co-terephthalate |
| PBSA | polybutylene succinate-co-adipate |
| PCL | poly(ε-caprolactone) |
| PEG | polyethylene glycol |
| PHB | poly(3-hydroxybutyrate) |
| phr | per hundred resin |
| PLA | polylactic acid |
| PLA/TPS | blend of 80 wt% PLA and 20 wt% TPS |
| ROP | ring-opening polymerization |
| Tcc | cold crystallization temperature |
| TGA | thermogravimetric analysis |
| TG | the weight loss versus temperature curves |
| Tg | glass transition temperature |
| Tmax | maximum degradation temperature |
| Tm | melting temperature |
| TPS | thermoplastic starch |
| T0 | degradation onset temperature |
| V | the volume of KOH used to titrate the sample |
| VOs | vegetable oils |
| w | the weight of the sample extracted from compost soil |
| wt% | parts by weight |
| w0 | initial dry weight of the sample |
| 2.5% MHO | PLA/TPS with the added 2.5 phr of MHO |
| 5% MHO | PLA/TPS with the added 5 phr of MHO |
| 7.5% MHO | PLA/TPS with the added 7.5 phr of MHO |
| 10% MHO | PLA/TPS with the added 10 phr of MHO |
References
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of Mechanical Ductile Properties of Poly(3-hydroxybutyrate) by Using Vegetable Oil Derivatives. Macromol. Mater. Eng. 2017, 302, 1600330. [Google Scholar] [CrossRef]
- Bioplastics—Facts and Figures. 2017. Available online: https://www.european-bioplastics.org (accessed on 30 March 2021).
- Carbonell-Verdu, A.; Garcia-Garcia, D.; Dominici, F.; Torre, L.; Sanchez-Nacher, L.; Balart, R. PLA films with improved flexibility properties by using maleinized cottonseed oil. Eur. Polym. J. 2017, 91, 248–259. [Google Scholar] [CrossRef]
- Arrieta, M.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological charac-terization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Matta, A.; Rao, R.U.; Suman, K.; Rambabu, V. Preparation and Characterization of Biodegradable PLA/PCL Polymeric Blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef] [Green Version]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 132. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Toughened Poly (Lactic Acid)—PLA Formulations by Binary Blends with Poly (Butylene Succinate-co-Adipate)—PBSA and Their Shape Memory Behaviour. Materials 2019, 12, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-M.; Lee, J.-W. Characterization and processing of biodegradable polymer blends of poly (lactic acid) with poly (butylene succinate adipate). Korea-Aust. Rheol. J. 2005, 17, 71–77. [Google Scholar]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Ferri, J.; Dominici, F.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Torre, L. Manufacturing and compat-ibilization of PLA/PBAT binary blends by cottonseed oil-based derivatives. Express Polym. Lett. 2018, 12, 808–823. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A new approach in compatibilization of the poly (lactic ac-id)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly (lactic acid) (PLA)/ thermoplastic starch (TPS) blends. Polym. Technol. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Müller, P.; Bere, J.; Fekete, E.; Móczó, J.; Nagy, B.; Kállay, M.; Gyarmati, B.; Pukánszky, B. Interactions, structure and properties in PLA/plasticized starch blends. Polymer 2016, 103, 9–18. [Google Scholar] [CrossRef]
- Shirai, M.; Grossmann, M.; Mali, S.; Yamashita, F.; Garcia, P.; Müller, C. Development of biodegradable flexible films of starch and poly (lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2010, 119, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Clasen, S.H.; Müller, C.M.O.; Pires, A.T.N. Maleic Anhydride as a Compatibilizer and Plasticizer in TPS/PLA Blends. J. Braz. Chem. Soc. 2015, 26, 1583–1590. [Google Scholar] [CrossRef]
- Chauhan, S.; Raghu, N.; Raj, A. Effect of maleic anhydride grafted polylactic acid concentration on mechanical and thermal properties of thermoplasticized starch filled polylactic acid blends. Polym. Polym. Compos. 2021, 09673911211004194. [Google Scholar] [CrossRef]
- Ferrarezi, M.M.F.; Taipina, M.D.O.; da Silva, L.C.E.; Gonçalves, M.D.C. Poly(Ethylene Glycol) as a Compatibilizer for Poly(Lactic Acid)/Thermoplastic Starch Blends. J. Polym. Environ. 2013, 21, 151–159. [Google Scholar] [CrossRef]
- Ozkoc, G.; Kemaloglu, S. Morphology, biodegradability, mechanical, and thermal properties of nanocomposite films based on PLA and plasticized PLA. J. Appl. Polym. Sci. 2009, 114, 2481–2487. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S. Effect of PEG on PLA/PEG blend and its nanocomposites: A study of thermo-mechanical and morphological characterization. Polym. Compos. 2014, 35, 283–293. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly (L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Rapa, M.; Nita, R.N.D.; Vasile, C. Influence of Plasticizers Over Some Physico-chemical Properties of PLA. Mater. Plast. 2017, 54, 73–78. [Google Scholar] [CrossRef]
- Burgos, N.; Tolaguera, D.; Fiori, S.; Jiménez, A. Synthesis and characterization of lactic acid oligomers: Evaluation of perfor-mance as poly (lactic acid) plasticizers. J. Polym. Environ. 2014, 22, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly (lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly (lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef] [Green Version]
- Oomah, B.; Busson, M.; Godfrey, D.V.; Drover, J.C. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by n-hexane. Ind. Crop. Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Liang, J.; Aachary, A.A.; Thiyam-Holländer, U. Hemp seed oil: Minor components and oil quality. Lipid Technol. 2015, 27, 231–233. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crop. Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, characterization and properties of hemp seed oil and its emulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, P.S.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Flexible bionanocomposites from epoxidized hemp seed oil ther-mosetting resin reinforced with halloysite nanotubes. J. Phys. Chem. B 2017, 121, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Carrillo, L.; Blanes-Martínez, M.; Montanes, N.; Fenollar, O.; Torres-Giner, S.; Balart, R. Reactive toughening of injec-tion-molded polylactide pieces using maleinized hemp seed oil. Eur. Polym. J. 2018, 98, 402–410. [Google Scholar] [CrossRef]
- Ferri, J.; Garcia-Garcia, D.; Sánchez-Nacher, L.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil (MLO) on mechanical performance of poly (lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohydr. Polym. 2016, 147, 60–68. [Google Scholar] [CrossRef]
- Ali, F.; Chang, Y.-W.; Kang, S.C.; Yoon, J.Y. Thermal, mechanical and rheological properties of poly (lactic acid)/epoxidized soybean oil blends. Polym. Bull. 2009, 62, 91–98. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. Preparation and characterization of poly (lactic acid) plasticized with vegetable oils and reinforced with sisal fibers. Ind. Crop. Prod. 2018, 112, 170–180. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.; López-Martínez, J.; Samper, M. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Jatropha Oil as a Sustainable Plasticizer to Poly (lactic Acid). Polymer 2017, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Abu Hassan, H.; Woei, C.B. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef] [Green Version]
- Ferri, J.M.; Garcia-Garcia, D.; Montanes, N.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil as biobased plasticizer in poly(lactic acid)-based formulations. Polym. Int. 2017, 66, 882–891. [Google Scholar] [CrossRef]
- Przybytek, A.; Sienkiewicz, M.; Kucińska-Lipka, J.; Janik, H. Preparation and characterization of biodegradable and com-postable PLA/TPS/ESO compositions. Ind. Crop. Prod. 2018, 122, 375–383. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Boix, A.C.; Malinconico, M.; Rippa, M.; di Serio, M.; Santagata, G.; et al. Poly (Lactic Acid)/Thermoplastic Starch Films: Effect of Cardoon Seed Epoxidized Oil on Their Chemicophysical, Mechanical, and Barrier Properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Meng, B.; Deng, J.; Liu, Q.; Wu, Z.; Yang, W. Transparent and ductile poly (lactic acid)/poly (butyl acrylate) (PBA) blends: Structure and properties. Eur. Polym. J. 2012, 48, 127–135. [Google Scholar] [CrossRef]
- Stein, W.J. Fettchemische und petrochemische Rohstoffe-Gegensatz oder Ergänzung? Fette Seifen Anstrichm. 1982, 84, 45–54. [Google Scholar] [CrossRef]
- Eren, T.; Wool, R. Polymerization of maleic anhydride-modified plant oils with polyols. J. Appl. Polym. Sci. 2003, 90, 197–202. [Google Scholar] [CrossRef]
- Teeter, H.M.; Geerts, M.J.; Cowan, J.C. Polymerization of drying oils. III. Some observations on reaction of maleic anhydride with methyl oleate and methyl linoleate. J. Am. Oil Chem. Soc. 1948, 25, 158–162. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, C.; Ma, S.; Feng, J.; Yang, Y.; Zhang, R.; Zhu, J. The properties of poly (lactic acid)/starch blends with a functionalized plant oil: Tung oil anhydride. Carbohydr. Polym. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Mauck, S.C.; Wang, S.; Ding, W.; Rohde, B.J.; Fortune, C.K.; Yang, G.; Ahn, S.-K.; Robertson, M.L. Biorenewable Tough Blends of Polylactide and Acrylated Epoxidized Soybean Oil Compatibilized by a Polylactide Star Polymer. Macromolecules 2016, 49, 1605–1615. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Chern, Y.C.; Tseng, S.M.; Hsieh, K.H. Damping properties of interpenetrating polymer networks of polyurethane-modified epoxy and polyurethanes. J. Appl. Polym. Sci. 1999, 74, 328–335. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Toschi, T.G. Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils. Molecules 2019, 24, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenia, V.; Toschi, T.G.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Minassi, A.; Fresu, L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar]
- Xiong, Z.; Yang, Y.; Feng, J.; Zhang, X.; Zhang, C.; Tang, Z.; Zhu, J. Preparation and characterization of poly (lactic acid)/starch composites toughened with epoxidized soybean oil. Carbohydr. Polym. 2013, 92, 810–816. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Samper, M.D.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Plasticization effect of epoxidized cottonseed oil (ECSO) on poly (lactic acid). Ind. Crop. Prod. 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Carlson, D.; Nie, L.; Narayan, R.; Dubois, P. Maleation of polylactide (PLA) by reactive extrusion. J. Appl. Polym. Sci. 1999, 72, 477–485. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Bhattacharya, M. Properties of blends of starch and synthetic polymers containing anhydride groups. J. Appl. Polym. Sci. 1994, 52, 617–628. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Sun, X. Mechanical Properties of Poly (lactic acid)/Starch Composites Compatibilized by Maleic Anhydride. Biomacromolecules 2004, 5, 1446–1451. [Google Scholar] [CrossRef]
- Mikus, P.-Y.; Alix, S.; Soulestin, J.; Lacrampe, M.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crop. Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.; Kittikorn, T.; Strömberg, E.; Martínez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal ageing of polylactide/sisal biocomposites. Studies of water absorption behaviour and Physico-Chemical performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Thakore, I.; Desai, S.; Sarawade, B.; Devi, S. Studies on biodegradability, morphology and thermo-mechanical properties of LDPE/modified starch blends. Eur. Polym. J. 2001, 37, 151–160. [Google Scholar] [CrossRef]








| Code | Parts by Weight (wt. %) | MHO (phr) | |
|---|---|---|---|
| PLA | TPS | ||
| PLA/TPS | 80 | 20 | - |
| PLA/TPS_2.5-MHO | 80 | 20 | 2.5 |
| PLA/TPS_5.0-MHO | 80 | 20 | 5.0 |
| PLA/TPS_7.5-MHO | 80 | 20 | 7.5 |
| PLA/TPS_10-MHO | 80 | 20 | 10.0 |
| Code | TGA Parameters | DSC Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| T0 (°C) | Tmax (°C) | Tg (°C) | Tm (°C) | ΔHm (J·g−1) | Tcc (°C) | ΔHcc (J·g−1) | Xc (%) | |
| PLA/TPS | 333 | 370.3 | 60.3 | 152.5 | 14.5 | 123.6 | 12.6 | 3.1 |
| PLA/TPS_2.5-MHO | 332 | 368.7 | 59.4 | 148.1 | 21.8 | 117.8 | 19.3 | 3.8 |
| PLA/TPS_5.0-MHO | 330 | 371.3 | 59.6 | 148.3 | 19.8 | 119.9 | 16.6 | 5.0 |
| PLA/TPS_7.5-MHO | 325 | 370.0 | 60.1 | 150.5 | 12.9 | 121.8 | 7.4 | 9.0 |
| PLA/TPS_10-MHO | 323 | 369.7 | 59.6 | 149.8 | 16.0 | 123.1 | 7.6 | 14.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerma-Canto, A.; Gomez-Caturla, J.; Herrero-Herrero, M.; Garcia-Garcia, D.; Fombuena, V. Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer. Polymers 2021, 13, 1392. https://doi.org/10.3390/polym13091392
Lerma-Canto A, Gomez-Caturla J, Herrero-Herrero M, Garcia-Garcia D, Fombuena V. Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer. Polymers. 2021; 13(9):1392. https://doi.org/10.3390/polym13091392
Chicago/Turabian StyleLerma-Canto, Alejandro, Jaume Gomez-Caturla, María Herrero-Herrero, Daniel Garcia-Garcia, and Vicent Fombuena. 2021. "Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer" Polymers 13, no. 9: 1392. https://doi.org/10.3390/polym13091392
APA StyleLerma-Canto, A., Gomez-Caturla, J., Herrero-Herrero, M., Garcia-Garcia, D., & Fombuena, V. (2021). Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer. Polymers, 13(9), 1392. https://doi.org/10.3390/polym13091392