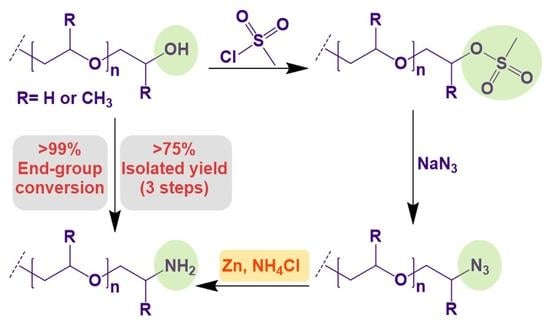
A Facile Strategy for the High Yielding, Quantitative Conversion of Polyglycol End-Groups to Amines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Procedures
2.3.1. Synthesis of Mesylate-Terminated Polyglycols
2.3.2. Synthesis of Azido-Terminated Polyglycols
2.3.3. Synthesis of Amino-Terminated Polyglycols
2.3.4. Sequential Reagent Addition Synthesis of α,ω-Diamino PEG1.5k
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, J.M. Poly (Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Springer Science & Business Media: New York, NY, USA, 1992. [Google Scholar]
- Albertsson, P.A. Partition of Cell Particles and Macromolecules: Separation and Purification of Biomolecules, Cell Organelles, Membranes, and Cells in Aqueous Polymer Two-Phase Systems and Their Use in Biochemical Analysis and Biotechnology; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Bhadra, D.; Bhadra, S.; Jain, P.; Jain, N. Pegnology: A review of PEG-ylated systems. Pharmazie 2002, 57, 5. [Google Scholar] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Watthey, J.W.; Stanton, J.L.; Desai, M.; Babiarz, J.E.; Finn, B.M. Synthesis and biological properties of (carboxyalkyl) amino-substituted bicyclic lactam inhibitors of angiotensin converting enzyme. J. Med. Chem. 1985, 28, 1511–1516. [Google Scholar] [CrossRef]
- Shieh, W.-C.; Carlson, J.A.; Zaunius, G.M. Asymmetric Synthesis of N-Substituted α-Aminobenzlactam via Crystallization-Induced Asymmetric Transformation of Covalent Diastereomer. J. Org. Chem. 1997, 62, 8271–8272. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Cobo, I.; Li, M.; Sumerlin, B.S.; Perrier, S. Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat. Mater. 2015, 14, 143. [Google Scholar] [CrossRef]
- Mero, A.; Clementi, C.; Veronese, F.M.; Pasut, G. Covalent conjugation of poly (ethylene glycol) to proteins and peptides: Strategies and methods. In Bioconjugation Protocols; Springer: New York, NY, USA, 2011; pp. 95–129. [Google Scholar]
- Greenwald, R.B.; Choe, Y.H.; McGuire, J.; Conover, C.D. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 2003, 55, 217–250. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Herman, S.; Hooftman, G.; Schacht, E. Poly (ethylene glycol) with reactive endgroups: I. Modification of proteins. J. Bioact. Compat. Polym. 1995, 10, 145–187. [Google Scholar] [CrossRef]
- Zalipsky, S. Functionalized poly (ethylene glycols) for preparation of biologically relevant conjugates. Bioconjugate Chem. 1995, 6, 150–165. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, S.H.; Lee, Y.M.; Chu, L.-Y. Biotin-conjugated block copolymeric nanoparticles as tumor-targeted drug delivery systems. Macromol. Res. 2007, 15, 646–655. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Kim, J.; Jung, H.Y.; Park, M.J. End-group chemistry and junction chemistry in polymer science: Past, present, and future. Macromolecules 2020, 53, 746–763. [Google Scholar] [CrossRef] [Green Version]
- Mutter, M. Soluble polymers in organic synthesis: I. Preparation of polymer reagents using polyethylene glycol with terminal amino groups as polymeric component. Tetrahedron Lett. 1978, 19, 2839–2842. [Google Scholar] [CrossRef]
- Harris, J.M. Laboratory synthesis of polyethylene glycol derivatives. J. Macromol. Sci. Part C 1985, 25, 325–373. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Bei, J.Z. Synthesis and characterization of macroinitiator-amino terminated PEG and poly (γ-benzyl-L-glutamate)-PEO-poly (γ-benzyl-L-glutamate) triblock copolymer. Polym. Adv. Technol. 2004, 15, 617–621. [Google Scholar] [CrossRef]
- Bückmann, A.F.; Morr, M.; Johansson, G. Functionalization of poly (ethylene glycol) and monomethoxy-poly (ethylene glycol). Makromol. Chem. 1981, 182, 1379–1384. [Google Scholar] [CrossRef]
- Mongondry, P.; Bonnans-Plaisance, C.; Jean, M.; Tassin, J.F. Mild Synthesis of Amino-Poly (ethylene glycol)s. Application to Steric Stabilization of Clays. Macromol. Rapid Commun. 2003, 24, 681–685. [Google Scholar] [CrossRef]
- Chua, G.B.; Roth, P.J.; Duong, H.T.; Davis, T.P.; Lowe, A.B. Synthesis and Thermoresponsive Solution Properties of Poly [oligo (ethylene glycol)(meth) acrylamide] s: Biocompatible PEG Analogues. Macromolecules 2012, 45, 1362–1374. [Google Scholar] [CrossRef]
- Brandl, F.; Henke, M.; Rothschenk, S.; Gschwind, R.; Breunig, M.; Blunk, T.; Teßmar, J.; Göpferich, A. Poly (ethylene glycol) based hydrogels for intraocular applications. Adv. Eng. Mater. 2007, 9, 1141–1149. [Google Scholar] [CrossRef]
- Duval, J.M.; Delestre, C.; Carré, M.-C.; Hubert, P.; Dellacherie, E. Synthesis and characterization of some covalent dextran-polyoxyethyleneglycol derivatives. Carbohydr. Polym. 1991, 15, 233–242. [Google Scholar]
- Zhou, C.; Truong, V.X.; Qu, Y.; Lithgow, T.; Fu, G.; Forsythe, J.S. Antibacterial poly (ethylene glycol) hydrogels from combined epoxy-amine and thiol-ene click reaction. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 656–667. [Google Scholar] [CrossRef]
- Zalipsky, S.; Gilon, C.; Zilkha, A. Attachment of drugs to polyethylene glycols. Eur. Polym. J. 1983, 19, 1177–1183. [Google Scholar] [CrossRef]
- Kugo, K.; Ohji, A.; Uno, T.; Nishino, J. Synthesis and conformations of ABA tri-block copolymers with hydrophobic poly (γ-benzyl l-glutamate) and hydrophilic poly (ethylene oxide). Polym. J. 1987, 19, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Luo, Z.; Zhou, D.; Cao, F.; Wang, Y. A novel PEG coating immobilized onto capillary through polydopamine coating for separation of proteins in CE. Electrophoresis 2010, 31, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Stefanko, M.J.; Gun’ko, Y.K.; Rai, D.K.; Evans, P. Synthesis of functionalised polyethylene glycol derivatives of naproxen for biomedical applications. Tetrahedron 2008, 64, 10132–10139. [Google Scholar] [CrossRef]
- Barrientos, Á.G.; Jesús, M.; Rojas, T.C.; Fernández, A.; Penadés, S. Gold glyconanoparticles: Synthetic polyvalent ligands mimicking glycocalyx-like surfaces as tools for glycobiological studies. Chem. Eur. J. 2003, 9, 1909–1921. [Google Scholar] [CrossRef]
- Banasik, B.; Nadala, C.; Samadpour, M. An Economical and Scalable Preparation of Poly (Ethylene Glycol) Methyl Ether Amine, MW 5,000. Org. Prep. Proced. Int. 2018, 50, 95–99. [Google Scholar] [CrossRef]
- Susumu, K.; Mei, B.C.; Mattoussi, H. Multifunctional ligands based on dihydrolipoic acid and polyethylene glycol to promote biocompatibility of quantum dots. Nat. Protoc. 2009, 4, 424. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, C.; Güner, A. Solubility profiles of poly (ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Batesky, D.C.; Goldfogel, M.J.; Weix, D.J. Removal of Triphenylphosphine Oxide by Precipitation with Zinc Chloride in Polar Solvents. J. Org. Chem. 2017, 82, 9931–9936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, N.G. Practical Process Research and Development: A Guide for Organic Chemists; Academic Press: Burlington, VT, USA, 2012. [Google Scholar]
- Comoy, C.; Fort, Y. Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hobken, NJ, USA, 2014. [Google Scholar]
- Abdollahi, H.; Salimi, A.; Barikani, M.; Zeynizadeh, B. New synthesis processes of polyetheramines: Comparison of three different developed amination routes. Mater. Manuf. Process. 2017, 32, 1296–1303. [Google Scholar] [CrossRef]
- Scriven, E.F.; Turnbull, K. Azides: Their preparation and synthetic uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar] [CrossRef]
- Bosch, I.; Costa, A.M.; Martín, M.; Urpi, F.; Vilarrasa, J. Reduction of azides to amines mediated by tin bis (1,2-benzenedithiolate). Org. Lett. 2000, 2, 397–399. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pizzo, F.; Vaccaro, L. Cobalt (II) chloride-catalyzed chemoselective sodium borohydride reduction of azides in water. Synthesis 2000, 2000, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.N.; Singh, M.P.; Micetich, R.G. Facile conversion of azides to amines. Tetrahedron Lett. 1986, 27, 1423–1424. [Google Scholar] [CrossRef]
- Kirk, D.; Wilson, M. A novel route to D-homoandrostane derivatives, including new methods for the preparation and reduction of hydroxy-azides. J. Chem. Soc. D Chem. Commun. 1970, 64b–65. Available online: https://pubs.rsc.org/en/content/articlelanding/1970/c2/c2970000064b/unauth#!divAbstract (accessed on 25 April 2021).
- Maiti, S.N.; Spevak, P.; Narender Reddy, A. Alkaline earth metal mediated reduction of azides to amines. Syn. Commun. 1988, 18, 1201–1206. [Google Scholar] [CrossRef]
- Adachi, T.; Yamada, Y.; Inoue, I.; Saneyoshi, M. An Alternative Method for the Selective Reduction of Unsaturated Nucleoside Azides to The Amines. Synthesis 1977, 45–46. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL7760191978 (accessed on 25 April 2021).
- Lin, W.; Zhang, X.; He, Z.; Jin, Y.; Gong, L.; Mi, A. Reduction of azides to amines or amides with zinc and ammonium chloride as reducing agent. Syn. Commun. 2002, 32, 3279–3284. [Google Scholar] [CrossRef]
- Hagenbuch, J.-P. Opportunities and limits of the use of azides in industrial production. Implementation of safety measures. Chimia 2003, 57, 773–776. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. 2005, 44, 5188–5240. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; Wiltshire, J.T.; Blencowe, A.; Qiao, G.G. Synthesis of a star polymer library with a diverse range of highly functionalized macromolecular architectures. Macromolecules 2011, 44, 3189–3202. [Google Scholar] [CrossRef]
- Meng, F.; Qiao, Z.; Yao, Y.; Luo, J. Synthesis of polyurethanes with pendant azide groups attached on the soft segments and the surface modification with mPEG by click chemistry for antifouling applications. RSC Adv. 2018, 8, 19642–19650. [Google Scholar] [CrossRef] [Green Version]



| Polymer | Isolated Yield (%) | End-Group Conversion (%) a | |||
|---|---|---|---|---|---|
| Mesylate | Azide | Amine | Overall | ||
| MeOPEG5k | 96.9 | 98.6 | 94.4 | 90.3 | >99 |
| PEG1.5k | 99.9 | 77.4 | 99.3 | 76.8 | >99 |
| PEG4.0k | 99.9 | 95.3 | 94.5 | 90.0 | >99 |
| PEG10k | 99.6 | 92.2 | 81.9 | 75.3 | >99 |
| PEG35k | 98.9 | 84.8 | 88.9 | 74.6 | >99 |
| 4-Armed PEG10k | 98.6 | 96.8 | 93.1 | 88.8 | >99 |
| PEG-PPG-PEG13k | 98.1 | 96.1 | 84.6 | 79.8 | >99 |
| nBuOPPG0.3k | 96.6 | 98.6 | 92.8 | 88.4 | >99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Marina, P.F.; Blencowe, A. A Facile Strategy for the High Yielding, Quantitative Conversion of Polyglycol End-Groups to Amines. Polymers 2021, 13, 1403. https://doi.org/10.3390/polym13091403
Yan J, Marina PF, Blencowe A. A Facile Strategy for the High Yielding, Quantitative Conversion of Polyglycol End-Groups to Amines. Polymers. 2021; 13(9):1403. https://doi.org/10.3390/polym13091403
Chicago/Turabian StyleYan, Jie, Paula Facal Marina, and Anton Blencowe. 2021. "A Facile Strategy for the High Yielding, Quantitative Conversion of Polyglycol End-Groups to Amines" Polymers 13, no. 9: 1403. https://doi.org/10.3390/polym13091403
APA StyleYan, J., Marina, P. F., & Blencowe, A. (2021). A Facile Strategy for the High Yielding, Quantitative Conversion of Polyglycol End-Groups to Amines. Polymers, 13(9), 1403. https://doi.org/10.3390/polym13091403