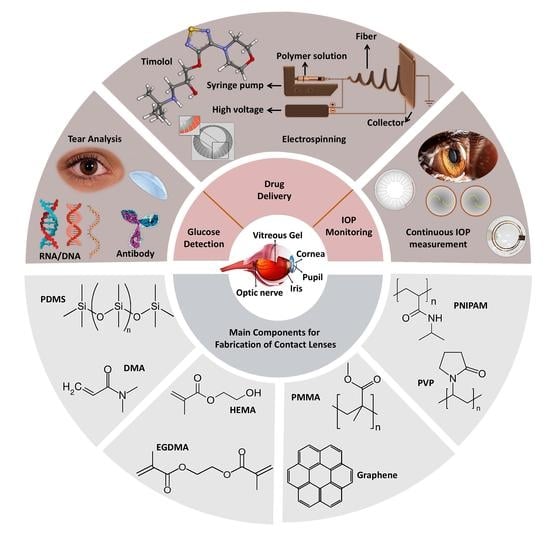
A Meta-Analysis of Wearable Contact Lenses for Medical Applications: Role of Electrospun Fiber for Drug Delivery
Abstract
:1. Introduction
2. Methodology
3. Contact Lenses for IOP Measurement
4. Contact Lenses for Glucose Detection
5. Contact Lenses for Colorblindness
6. Contact Lenses for Drug Delivery
7. Electrospun-Fiber-Incorporated Contact Lenses for Drug Delivery
8. Limitations and Existing Challenges of the Contact Lenses
9. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-APBA | 3-(acrylamido)phenylboronic acid |
| AA | Acrylamide |
| AAO | Anodic aluminum oxide |
| Abs | Antibodies |
| AgNW | Graphene–silver nanowire |
| Ags | Antigens |
| AIBN | Azoisobutyronitrile |
| AIPH | 2-20-azobis [2-(2-imidazolin-2-yl) propane] dihydrochloride |
| ASIC | Application-specific integrated circuit |
| ASK | Amplitude shift keying |
| AVR | Alf Vegard Risc |
| BAC | Benzalkonium chloride |
| BAFs | Boronic-acid-containing fluorophores |
| BIS | N,N-methylenebis(acrylamide) |
| BL | Bi-layer |
| BMCL | Bicontinuous microemulsion contact lens |
| BME-CLs | Bicontinuous microemulsion nanoporous contact lenses |
| BOE | Buffered oxide etchant |
| BOTE | Back of the eye |
| BT-SM | Bimatoprost-soaked contact lenses |
| CCA | Crystalline colloidal array |
| C-HA | Cholesterol–hyaluronate |
| CLS | Contact lens sensor |
| CMOS | Complementary metal-oxide semiconductor |
| CNTs | Carbon nanotubes |
| CS | Chitosan |
| CVD | Color vision deficiency |
| DEAA | N,N-diethylacrylamide |
| DEAP | 2,2-diethoxyacetophenone |
| DED | Dry eye disease |
| DES | Dry eye syndrome |
| DI water | Deionized water |
| DCM | Dichloromethane |
| DMA | Dimethyl acrylamide |
| DMAA | N,N-dimethylacrylamide |
| DMSO | Dimethylsulfoxide |
| DRIE | Deep-reactive-ion etching |
| EA | Ethanolamine |
| EDC | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| EGDMA | Ethylene glycol dimethacrylate |
| EGF | Epithelial growth factor |
| EHD | Electrohydrodynamic |
| EHDA | Electrohydrodynamic atomization |
| EIRP | Effective isotropically radiated power |
| f-DDS | Flexible drug delivery system |
| FDA | Food and Drug Administration |
| FOTE | Front of the eye |
| FSS | Fluorescein sodium salt |
| FITC-dextran | Fluorescein isothiocyanate–dextran |
| GAT | Goldmann applanation tonometer |
| GMA | Glycidyl methacrylate |
| GO | Graphene oxide |
| GOD | Glucose oxidase |
| HA | Hyaluronic acid |
| HEMA | Hydroxyethyl methacrylate |
| HOG | Histogram of gradient |
| HTCC | Quaternized chitosan |
| HMPP | 2-hydroxy-2-methyl-1-phenyl-1-propanone |
| IC | Integrated circuit |
| IL-12p | Interleukin 12 |
| IOP | Intraocular pressure |
| ITES | Implantable Telemetric Endosystem |
| LC | Inductive coil with a capacitor |
| LED | Light-emitting diode |
| MNPC | Magnetic nanoparticle–PDMS composite |
| MA | Methacrylic acid |
| MEMS | Micro-electromechanical systems |
| mNWs | Metal nanowires |
| MoS2 | Molybdenum disulfide |
| NHS | N-Hydroxysuccinimide |
| NIPAM | N-isopropylacrylamide |
| NOA65 | Norland Optical Adhesive 65 |
| NVP | N-vinyl pyrrolidone |
| OL | Olopatadine HCl |
| OPA | Ocular pulse amplitude |
| OTS | Ocular telemetry sensor |
| PBS | Phosphate-buffered saline |
| PC | Polycarbonate |
| PCL | Poly ε-caprolactone |
| PDMS | Polydimethylsiloxane |
| PEG-DA | Poly ethylene glycol diacrylate |
| PGT | Propoxylated glyceryl triacylate |
| PVA | Poly (vinyl alcohol) |
| PE | Borneol |
| PEs | Permeation enhancers |
| PET | Polyethylene terephthalate |
| PETE | Polyethylene terephthalate |
| PLGA | Poly(lactic-co-glycolic acid) |
| pHEMA | Poly(2-hydroxyethyl methacrylate) |
| PI | Polyimide |
| PMCL | Pressure-measuring contact lens |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PPA | Phosphoric acid |
| PS | Photonic structure |
| Pt | Platinum |
| PtITi | Platinum–titanium |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinyl pyrrolidone |
| RIE | Reactive ion etching |
| RGP | Rigid gas permeable |
| Sp | Sparfloxacin |
| SiO2 | Silica |
| SNP | Spherical silver nanoparticles |
| STF | Simulated tear fluid |
| STZ | Streptozotocin |
| TBA | Tetrabutylammonium |
| TRITC-Con A | Tetramethylrhodamine isothiocyanate–concanavalin A |
| Ti | Titanium |
| TM | Timolol maleate |
| TMDC | Transition metal dichalcogenide |
| UV | Ultraviolet |
| Vor | Voriconazole |
| WHO | World Health Organization |
| ZIF | Zero Insertion Force |
| µTM | Microtransfer molding |
References
- Leonardi, M.; Leuenberger, P.; Bertrand, D.; Bertsch, A.; Renaud, P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3113–3117. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, G.; Maleki, T.; Samuels, B.C.; Cantor, L.B.; Ziaie, B. Minimally Invasive Implantable Wireless Pressure Sensor for Continuous IOP Monitoring. Investig. Ophthalmol. Vis. Sci. 2011, 52, 666. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Y.; Wang, X.; Qi, C.; Yang, Y.; Ren, T.-L. A contact lens promising for non-invasive continuous intraocular pressure monitoring. RSC Adv. 2019, 9, 5076–5082. [Google Scholar] [CrossRef] [Green Version]
- Laukhin, V.; Sánchez, I.; Moya, A.; Laukhina, E.; Martin, R.; Ussa, F.; Rovira, C.; Guimera, A.; Villa, R.; Aguiló, J. Non-invasive intraocular pressure monitoring with a contact lens engineered with a nanostructured polymeric sensing film. Sens. Actuators A Phys. 2011, 170, 36–43. [Google Scholar] [CrossRef]
- Chen, G.-Z.; Chan, I.-S.; Leung, L.K.; Lam, D.C. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med. Eng. Phys. 2014, 36, 1134–1139. [Google Scholar] [CrossRef]
- Chen, G.-Z.; Chan, I.-S.; Lam, D.C. Capacitive contact lens sensor for continuous non-invasive intraocular pressure monitoring. Sens. Actuators A Phys. 2013, 203, 112–118. [Google Scholar] [CrossRef]
- Boehm, F. Nanomedical Device and Systems Design: Challenges, Possibilities, Visions; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kouhani, M.H.M.; Wu, J.; Tavakoli, A.; Weber, A.J.; Li, W. Wireless, passive strain sensor in a doughnut-shaped contact lens for continuous non-invasive self-monitoring of intraocular pressure. Lab Chip 2020, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Goedkoop, R.; Weinreb, R.N. A Minimally Invasive Device for the Monitoring of 24-hour Intraocular Pressure Patterns US. Ophthalmic Rev. 2013, 6, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Ben-Shlomo, G.; Que, L. A Multifunctional Smart Soft Contact Lens Device Enabled by Nanopore Thin Film for Glaucoma Diagnostics and In Situ Drug Delivery. J. Microelectromech. Syst. 2019, 28, 810–816. [Google Scholar] [CrossRef]
- Wasilewicz, R.; Varidel, T.; Simon-Zoula, S.; Schlund, M.; Cerboni, S.; Mansouri, K. First-in-human continuous 24-hour measurement of intraocular pressure and ocular pulsation using a novel contact lens sensor. Br. J. Ophthalmol. 2020, 104, 1519–1523. [Google Scholar] [CrossRef]
- Otis, B.; Parviz, B. Introducing Our Smart Contact Lens Project; Google LLC: Mountain View, CA, USA, 2014. [Google Scholar]
- Senior, M. Novartis Signs Up for Google Smart Lens; Nature Publishing Group: Berlin, Germany, 2014. [Google Scholar]
- Kim, S.-K.; Koo, J.; Lee, G.-H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.-K.; Shin, S. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef] [Green Version]
- Newman-Casey, P.A.; Robin, A.L.; Blachley, T.; Farris, K.; Heisler, M.; Resnicow, K.; Lee, P.P. The most common barriers to glaucoma medication adherence: A cross-sectional survey. Ophthalmology 2015, 122, 1308–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.-C.; Ben-Shlomo, A.; Mackay, E.O.; Plummer, C.E.; Chauhan, A. Drug delivery by contact lens in spontaneously glaucomatous dogs. Curr. Eye Res. 2012, 37, 204–211. [Google Scholar] [CrossRef]
- Ding, X.; Song, C.; Que, L. Fabrication of Contact Lens Device with Integrated Microtubes for In Situ Extended Drug Delivery for Ocular Disease Treatment, Proceedings of 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 306–309. [Google Scholar]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; van Dijck, A.; Mensch, J.; Noppe, M.; E, M. Brewster, Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release 2003, 92, 349–360. [Google Scholar] [CrossRef]
- Fuerst, R.; Bango, J.; Fenn, J.; Dziekan, M. Fabrication of Improved Contact Lens Utilizing Polymer Electrospinning. Google Patents. U.S. Patent No. 7,563,396, 21 July 2009. [Google Scholar]
- Davis, K.; Reuter, M.; Kammerich, A.; Tangonan, A.; Vedantham, K.; Kelley, A. Extended Release Drug-Delivery Contact Lenses and Methods of Making. Google Patents. U.S. Patent No. 9,956,168, 1 May 2018. [Google Scholar]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Chang, M.-W.; Alany, R.G.; Ahmad, Z. Development and characterisation of electrospun timolol maleate-loaded polymeric contact lens coatings containing various permeation enhancers. Int. J. Pharm. 2017, 532, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Development of novel tissue engineering scaffolds via electrospinning. Exp. Opin. Biol. Ther. 2004, 4, 659–668. [Google Scholar] [CrossRef]
- Göttel, B.; de Souza e Silva, J.M.; de Oliveira, C.S.; Syrowatka, F.; Fiorentzis, M.; Viestenz, A.; Viestenz, A.; Mäder, K. Electrospun nanofibers–A promising solid in-situ gelling alternative for ocular drug delivery. Eur. J. Pharm. Biopharm. 2020, 146, 125–132. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Singh, N.; van der Merwe, S.M.; Chang, M.-W.; Alany, R.G.; Ahmad, Z. Engineering and development of chitosan-based Nanocoatings for Ocular Contact Lenses. J. Pharm. Sci. 2019, 108, 1540–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Al-Kinani, A.A.; Qutachi, O.; Arshad, M.S.; Alqahtani, A.; Chang, M.-W.; Amoaku, W.M.; Alany, R.G.; Ahmad, Z. Assessing the ex vivo permeation behaviour of functionalised contact lens coatings engineered using an electrohydrodynamic technique. J. Phys. Mater. 2018, 2, 014002. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Haj-Ahmad, R.; Arshad, M.S.; Chang, M.-W.; Alany, R.G.; Ahmad, Z. Electrically atomised formulations of timolol maleate for direct and on-demand ocular lens coatings. Eur. J. Pharm. Biopharm. 2017, 119, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.; Ghaboussi, J.; Pecknold, D.; Hashash, Y.M. Effect of cornea material stiffness on measured intraocular pressure. J. Biomech. 2008, 41, 1707–1713. [Google Scholar] [CrossRef]
- Doughty, M.J.; Jonuscheit, S. Effect of central corneal thickness on Goldmann applanation tonometry measures—A different result with different pachymeters. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1603–1610. [Google Scholar] [CrossRef]
- Liu, J. Circadian rhythm of intraocular pressure. J. Glaucoma 1998, 7, 141–147. [Google Scholar] [CrossRef]
- Liu, J.; Kripke, D.F.; Hoffman, R.E.; Twa, M.D.; Loving, R.T.; Rex, K.M.; Gupta, N.; Weinreb, R.N. Nocturnal elevation of intraocular pressure in young adults. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2707–2712. [Google Scholar]
- Leonardi, M.; Leuenberger, P.; Bertrand, D.; Bertsch, A.; Renaud, P. A Soft Contact Lens with a MEMS Strain Gage Embedded for Intraocular Pressure Monitoring, Proceedings of TRANSDUCERS’03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; Digest of Technical Papers (Cat. No. 03TH8664); IEEE: Piscataway, NJ, USA, 2003; pp. 1043–1046. [Google Scholar]
- Twa, M.D.; Roberts, C.J.; Karol, H.J.; Mahmoud, A.M.; Weber, P.A.; Small, R.H. Evaluation of a contact lens-embedded sensor for intraocular pressure measurement. J. Glaucoma 2010, 19, 382. [Google Scholar] [CrossRef] [Green Version]
- Chitnis, G.; Maleki, T.; Samuels, B.; Cantor, L.B.; Ziaie, B. A minimally invasive implantable wireless pressure sensor for continuous IOP monitoring. IEEE Trans. Biomed. Eng. 2012, 60, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Saati, S.; Varma, R.; Humayun, M.S.; Tai, Y.-C. Implantable Flexible-Coiled Wireless Intraocular Pressure Sensor, Proceedings of 2009 IEEE 22nd International Conference on Micro Electro Mechanical Systems, Sorrento, Italy, 25–29 January 2009; IEEE: Piscataway, NJ, USA, 2009. [Google Scholar]
- Greene, M.; Gilman, B. Intraocular pressure measurement with instrumented contact lenses. Investig. Ophthalmol. Vis. Sci. 1974, 13, 299–302. [Google Scholar]
- Araci, I.E.; Su, B.; Quake, S.R.; Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 2014, 20, 1074. [Google Scholar] [CrossRef]
- Leonardi, M.; Pitchon, E.M.; Bertsch, A.; Renaud, P.; Mermoud, A. Wireless contact lens sensor for intraocular pressure monitoring: Assessment on enucleated pig eyes. Acta Ophthalmol. 2009, 87, 433–437. [Google Scholar] [CrossRef]
- Mansouri, K.; Shaarawy, T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: Initial clinical experience in patients with open angle glaucoma. Br. J. Ophthalmol. 2011, 95, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, K.-S.; Kim, J.-W.; Kang, J.-Y.; Kim, J.-K. Stimulus-Responsive Contact Lens for IOP Measurement or Temperature-Triggered Drug Release. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campigotto, A.; Leahy, S.; Zhao, G.; Campbell, R.J.; Lai, Y. Non-invasive Intraocular pressure monitoring with contact lens. Br. J. Ophthalmol. 2020, 104, 1324–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araci, I.E.; Agaoglu, S.; Baday, M.; Diep, P. Closed Microfluidic Network for Strain Sensing Embedded in a Contact Lens to Monitor IntraOcular Pressure. Google Patents. U.S. Patent No. 10,898,074, 26 January 2021. [Google Scholar]
- Maeng, B.; Chang, H.-K.; Park, J. Photonic crystal-based smart contact lens for continuous intraocular pressure monitoring. Lab Chip 2020, 20, 1740–1750. [Google Scholar] [CrossRef]
- Agaoglu, S.; Diep, P.; Martini, M.; Samudhyatha, K.; Baday, M.; Araci, I.E. Ultra-sensitive microfluidic wearable strain sensor for intraocular pressure monitoring. Lab Chip 2018, 18, 3471–3483. [Google Scholar] [CrossRef]
- Xu, J.; Cui, T.; Hirtz, T.; Qiao, Y.; Li, X.; Zhong, F.; Han, X.; Yang, Y.; Zhang, S.; Ren, T.-L. Highly Transparent and Sensitive Graphene Sensors for Continuous and Non-invasive Intraocular Pressure Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 18375–18384. [Google Scholar] [CrossRef]
- Baena-Díez, J.M.; Peñafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marín-Ibañez, A.; Guembe, M.J.; Rigo, F.; Tormo-Díaz, M.J.; Moreno-Iribas, C. Risk of cause-specific death in individuals with diabetes: A competing risks analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Mathers, C.; Stevens, G.; Mascarenhas, M. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Fernandes, J.d.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.; Makaroff, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Krug, E.G. Trends in diabetes: Sounding the alarm. Lancet 2016, 387, 1485–1486. [Google Scholar] [CrossRef] [Green Version]
- Atlas, D. International Diabetes Federation, IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Olansky, L.; Kennedy, L. Finger-stick glucose monitoring: Issues of accuracy and specificity. Diabetes Care 2010, 33, 948–949. [Google Scholar] [CrossRef] [Green Version]
- Haxha, S.; Jhoja, J. Optical based noninvasive glucose monitoring sensor prototype. IEEE Photonics J. 2016, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Anas, M.N.; Nurun, N.; Norali, A.; Normahira, M. Non-Invasive Blood Glucose Measurement, Proceedings of 2012 IEEE-EMBS Conference on Biomedical Engineering and Sciences, Langkawi, Malaysia, 17–19 December 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 503–507. [Google Scholar]
- Asaduzzaman, A.; Samadarsinee, S.; Chidella, K.K. Simulating Multisensor Noninvasive Blood Glucose Monitoring Systems, Proceedings of the SoutheastCon 2016, Norfolk, VA, USA, 30 March–3 April 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–7. [Google Scholar]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Festin, P.J.F.; Cortez, R.S.; Villaverde, J.F. Non-Invasive Detection of Diabetes Mellitus by Tongue Diagnosis Using Convolutional Neural Network, Proceedings of the 2020 10th International Conference on Biomedical Engineering and Technology, Tokyo, Japan, 15–18 September 2020; Association for Computing Machinery: New York, NY, USA, 2020; pp. 135–139. [Google Scholar]
- Ma, M.; Zhou, Y.; Li, J.; Ge, Z.; He, H.; Tao, T.; Cai, Z.; Wang, X.; Chang, G.; He, Y. Non-invasive detection of glucose via a solution-gated graphene transistor. Analyst 2020, 145, 887–896. [Google Scholar] [CrossRef]
- Norman, J.J.; Brown, M.R.; Raviele, N.A.; Prausnitz, M.R.; Felner, E.I. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2013, 14, 459–465. [Google Scholar] [CrossRef]
- Hasan, K.U.; Asif, M.H.; Hassan, M.U.; Sandberg, M.O.; Nur, O.; Willander, M.; Fagerholm, S.; Strålfors, P. A miniature graphene-based biosensor for intracellular glucose measurements. Electrochim. Acta 2015, 174, 574–580. [Google Scholar] [CrossRef]
- Chu, M.X.; Miyajima, K.; Takahashi, D.; Arakawa, T.; Sano, K.; Sawada, S.-I.; Kudo, H.; Iwasaki, Y.; Akiyoshi, K.; Mochizuki, M. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 2011, 83, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact Lens Sensors in Ocular Diagnostics. Adv. Healthc. Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aass, C.; Norheim, I.; Eriksen, E.F.; Thorsby, P.M.; Pepaj, M. Single unit filter-aided method for fast proteomic analysis of tear fluid. Anal. Biochem. 2015, 480, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Aluru, S.V.; Agarwal, S.; Srinivasan, B.; Iyer, G.K.; Rajappa, S.M.; Tatu, U.; Padmanabhan, P.; Subramanian, N.; Narayanasamy, A. Lacrimal proline rich 4 (LPRR4) protein in the tear fluid is a potential biomarker of dry eye syndrome. PLoS ONE 2012, 7, e51979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheis, N.; Grus, F.H.; Breitenfeld, M.; Knych, I.; Funke, S.; Pitz, S.; Ponto, K.A.; Pfeiffer, N.; Kahaly, G.J. Proteomics differentiate between thyroid-associated orbitopathy and dry eye syndrome. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2649–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torok, Z.; Peto, T.; Csosz, E.; Tukacs, E.; Molnar, A.; Maros-Szabo, Z.; Berta, A.; Tozser, J.; Hajdu, A.; Nagy, V. Tear fluid proteomics multimarkers for diabetic retinopathy screening. BMC Ophthalmol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Patnaik, K.; Pradeep, A.; Nagpal, K.; Karvekar, S.; Singh, P.; Raju, A. Human chemerin correlation in gingival crevicular fluid and tear fluid as markers of inflammation in chronic periodontitis and type-2 diabetes mellitus. J. Investig. Clin. Dent. 2017, 8, e12181. [Google Scholar] [CrossRef]
- Leonardi, A.; Palmigiano, A.; Mazzola, E.; Messina, A.; Milazzo, E.; Bortolotti, M.; Garozzo, D. Identification of human tear fluid biomarkers in vernal keratoconjunctivitis using iTRAQ quantitative proteomics. Allergy 2014, 69, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wei, R.; Zhao, P.; Koh, S.K.; Beuerman, R.W.; Ding, C. Proteomic analysis revealed the altered tear protein profile in a rabbit model of S jögren’s syndrome-associated dry eye. Proteomics 2013, 13, 2469–2481. [Google Scholar] [CrossRef] [Green Version]
- Coyle, P.; Sibony, P.; Johnson, C. Oligoclonal IgG in tears. Neurology 1987, 37, 853. [Google Scholar] [CrossRef]
- Dikovskaya, M.A.; Trunov, A.N.; Chernykh, V.V.; Korolenko, T.A. Cystatin C and lactoferrin concentrations in biological fluids as possible prognostic factors in eye tumor development. Int. J. Circumpolar Health 2013, 72, 21087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrecht, A.; Boehm, D.; Schmidt, M.; Koelbl, H.; Schwirz, R.L.; Grus, F.H. Diagnosis of breast cancer by tear proteomic pattern. Cancer Genom. Proteom. 2009, 6, 177–182. [Google Scholar]
- Böhm, D.; Keller, K.; Pieter, J.; Boehm, N.; Wolters, D.; Siggelkow, W.; Lebrecht, A.; Schmidt, M.; Kölbl, H.; Pfeiffer, N. Comparison of tear protein levels in breast cancer patients and healthy controls using a de novo proteomic approach. Oncol. Rep. 2012, 28, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Stolwijk, T.R.; Kuizenga, A.; van Haeringen, N.J.; Kijlstra, A.; Oosterhuis, J.A.; van Best, J.A. Analysis of tear fluid proteins in insulin-dependent diabetes mellitus. Acta Ophthalmol. 1994, 72, 357–362. [Google Scholar] [CrossRef]
- Li, B.; Sheng, M.; Xie, L.; Liu, F.; Yan, G.; Wang, W.; Lin, A.; Zhao, F.; Chen, Y. Tear proteomic analysis of patients with type 2 diabetes and dry eye syndrome by two-dimensional nano-liquid chromatography coupled with tandem mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2014, 55, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Fullard, R.J.; Tucker, D. Tear Protein Composition and the Effects of Stimulus, Lacrimal Gland, Tear Film, and Dry Eye Syndromes; Springer: Boston, MA, USA, 1994; pp. 309–314. [Google Scholar]
- De Souza, G.A.; de Godoy, L.M.; Mann, M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006, 7, R72. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, N.; Zheng, J.; Liu, X.M.; Lever, O.W.; Erickson, P.M.; Li, L. Characterization of human tear proteome using multiple proteomic analysis techniques. J. Proteome Res. 2005, 4, 2052–2061. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3-μW CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid-State Circuits 2011, 47, 335–344. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.-Y.; Cheong, W.H.; Jang, J.; Park, Y.-G.; Na, K.; Kim, Y.-T.; Heo, J.H.; Lee, C.Y. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Wu, K.; Li, C.; Wang, H.; Sun, Z.; Xi, D.; Zhang, S.; Ding, W.; Zaghloul, M.E.; Wang, C. Integrated contact lens sensor system based on multifunctional ultrathin MoS2 transistors. Matter 2021, 4, 969–985. [Google Scholar] [CrossRef]
- Moreddu, R.; Elsherif, M.; Adams, H.; Moschou, D.; Cordeiro, M.F.; Wolffsohn, J.S.; Vigolo, D.; Butt, H.; Cooper, J.M.; Yetisen, A.K. Integration of paper microfluidic sensors into contact lenses for tear fluid analysis. Lab Chip 2020, 20, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A gelated colloidal crystal attached lens for noninvasive continuous monitoring of tear glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, L.T.; Stockman, A.; Jägle, H.; Nathans, J. Opsin genes, cone photopigments, color vision, and color blindness. In Color Vision: From Genes to Perception; Cambridge University Press: Cambridge, UK, 1999; pp. 3–51. [Google Scholar]
- Albany-Ward, K. What do you really know about colour blindness? Br. J. Sch. Nurs. 2015, 10, 197–199. [Google Scholar] [CrossRef]
- Jägle, H.; de Luca, E.; Serey, L.; Bach, M.; Sharpe, L.T. Visual acuity and X-linked color blindness. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 447–453. [Google Scholar] [CrossRef]
- Simunovic, M. Colour vision deficiency. Eye 2010, 24, 747–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schornack, M.M.; Brown, W.L.; Siemsen, D.W. The use of tinted contact lenses in the management of achromatopsia. Optom. J. Am. Optom. Assoc. 2007, 78, 17–22. [Google Scholar] [CrossRef]
- Salih, A.E.; Elsherif, M.; Ali, M.; Vahdati, N.; Yetisen, A.K.; Butt, H. Ophthalmic Wearable Devices for Color Blindness Management. Adv. Mater. Technol. 2020, 5, 1901134. [Google Scholar] [CrossRef]
- Gómez-Robledo, L.; Valero, E.; Huertas, R.; Martínez-Domingo, M.; Hernández-Andrés, J. Do EnChroma glasses improve color vision for colorblind subjects? Opt. Express 2018, 26, 28693–28703. [Google Scholar] [CrossRef]
- Ruminski, J. Color processing for color-blind individuals using smart glasses. J. Med. Imag. Health Inform. 2015, 5, 1652–1661. [Google Scholar] [CrossRef]
- Lee, J.; Anki, C.; Juyeon, S. Electronic Glasses and Method for Correcting Color Blindness. Google Patents. U.S. Patent No. 10,025,098, 17 July 2018. [Google Scholar]
- Oriowo, O.M.; Alotaibi, A.Z. Chromagen lenses and abnormal colour perception. Afr. Vis. Eye Health 2011, 70, 69–74. [Google Scholar] [CrossRef]
- Zielinski, W.L.; Sullivan, T.R. Ophthalmic drug therapy—Challenges and advances in front-of-the-eye delivery. Drug Deliv. 2007, 2, 309–323. [Google Scholar]
- Mainardes, R.M.; Urban, M.C.; Cinto, P.O.; Khalil, N.M.; Chaud, M.V.; Evangelista, R.C.; Gremiao, M.P.D. Colloidal carriers for ophthalmic drug delivery. Curr. Drug Targets 2005, 6, 363–371. [Google Scholar] [CrossRef]
- Davies, N.M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 2000, 27, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Dhake, A.S.; Sharma, S.K.; Majumdar, D.K. Topical ocular delivery of NSAIDs. AAPS J. 2008, 10, 229. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Rawas-Qalaji, M.; Williams, C.-A. Advances in ocular drug delivery. Curr. Eye Res. 2012, 37, 345–356. [Google Scholar] [CrossRef]
- Huang, J.-F.; Zhong, J.; Chen, G.-P.; Lin, Z.-T.; Deng, Y.; Liu, Y.-L.; Cao, P.-Y.; Wang, B.; Wei, Y.; Wu, T. A hydrogel-based hybrid theranostic contact lens for fungal keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef]
- Peng, C.-C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef]
- Rad, M.S.; Mohajeri, S.A. Extended ciprofloxacin release using vitamin E diffusion barrier from commercial silicone-based soft contact lenses. Eye Contact Lens 2017, 43, 103–109. [Google Scholar]
- Dixon, P.; Shafor, C.; Gause, S.; Hsu, K.-H.; Powell, K.C.; Chauhan, A. Therapeutic contact lenses: A patent review. Expert Opin. Ther. Pat. 2015, 25, 1117–1129. [Google Scholar] [CrossRef]
- Carvalho, I.; Marques, C.; Oliveira, R.; Coelho, P.; Costa, P.; Ferreira, D. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shaikh, A.A.; Lakdawala, D.H.; Desai, A.R.; Pandya, M.M.; Singhania, S.S.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Design and optimization of a novel implantation technology in contact lenses for the treatment of dry eye syndrome: In vitro and in vivo evaluation. Acta Biomater. 2017, 53, 211–221. [Google Scholar] [CrossRef]
- Mun, J.; Mok, J.W.; Jeong, S.; Cho, S.; Joo, C.-K.; Hahn, S.K. Drug-eluting contact lens containing cyclosporine-loaded cholesterol-hyaluronate micelles for dry eye syndrome. RSC Adv. 2019, 9, 16578–16585. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Liu, Y.; Han, S.; Zhao, Q.; Liu, N. Bimatoprost Imprinted Silicone Contact Lens to Treat Glaucoma. AAPS PharmSciTech 2020, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.J.; Kim, D.H.; Chang, W.S.; Vales, T.P.; Kim, J.W.; Kim, K.H.; Kim, J.K. Thermo-sensitive nanogel-laden bicontinuous microemulsion drug-eluting contact lenses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release. Mater. Sci. Eng. C 2020, 112, 110885. [Google Scholar] [CrossRef]
- Ran, W.; Ma, H.; Li, M. In Vitro and In Vivo Studies of Polyvinyl Pyrrolidone—Coated Sparfloxacin-Loaded Ring Contact Lens to Treat Conjunctivitis. J. Pharm. Sci. 2020, 109, 1951–1957. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, W.; Lei, Y.; Dang, M. Novel Polyvinyl Pyrrolidone—Loaded Olopatadine HCl-Laden Doughnut Contact Lens to Treat Allergic Conjunctivitis. J. Pharm. Sci. 2020, 109, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- ElShaer, A.; Mustafa, S.; Kasar, M.; Thapa, S.; Ghatora, B.; Alany, R.G. Nanoparticle-laden contact lens for controlled ocular delivery of prednisolone: Formulation optimization using statistical experimental design. Pharmaceutics 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Chen, S.; Chen, J.; Ding, H.; Deng, D.; Xie, Z. Self-reporting colorimetric analysis of drug release by molecular imprinted structural color contact lens. ACS Appl. Mater. Interfaces 2018, 10, 34611–34617. [Google Scholar] [CrossRef]
- Wang, C.; Park, J. Magnetic micropump embedded in contact lens for on-demand drug delivery. Micro Nano Syst. Lett. 2020, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Srinivasan, G.; Reneker, D.H. Structure and morphology of small diameter electrospun aramid fibers. Polym. Int. 1995, 36, 195–201. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Wang, Y.; Santiago-Avilés, J.J. Large negative magnetoresistance and two-dimensional weak localization in carbon nanofiber fabricated using electrospinning. J. Appl. Phys. 2003, 94, 1721–1727. [Google Scholar] [CrossRef]
- Kaplan, J.A.; Liu, R.; Freedman, J.D.; Padera, R.; Schwartz, J.; Colson, Y.L.; Grinstaff, M.W. Prevention of lung cancer recurrence using cisplatin-loaded superhydrophobic nanofiber meshes. Biomaterials 2016, 76, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Liu, Y.; Yu, Y.; Li, J.; Luo, J.; Li, M. Silk fibroin nanoparticles prepared by electrospray as controlled release carriers of cisplatin. Mater. Sci. Eng. C 2014, 44, 166–174. [Google Scholar] [CrossRef]
- Gao, Y.; Teoh, T.W.; Wang, Q.; Williams, G.R. Electrospun organic–inorganic nanohybrids as sustained release drug delivery systems. J. Mater. Chem. B 2017, 5, 9165–9174. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, B.; Wang, Y.; Lou, D. Polymer-controlled core–shell nanoparticles: A novel strategy for sequential drug release. RSC Adv. 2014, 4, 30430–30439. [Google Scholar] [CrossRef]
- Wang, J.-C.; Zheng, H.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Preparation of active 3D film patches via aligned fiber electrohydrodynamic (EHD) printing. Sci. Rep. 2017, 7, 43924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasekh, M.; Ahmad, Z.; Cross, R.; Hernández-Gil, J.; Wilton-Ely, J.D.; Miller, P.W. Facile preparation of drug-loaded tristearin encapsulated superparamagnetic iron oxide nanoparticles using coaxial electrospray processing. Mol. Pharm. 2017, 14, 2010–2023. [Google Scholar] [CrossRef] [Green Version]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.-P.N. Electrosprayed mesoporous particles for improved aqueous solubility of a poorly water soluble anticancer agent: In vitro and ex vivo evaluation. J. Control. Release 2018, 278, 142–155. [Google Scholar] [CrossRef]
- Lingley, A.R.; Ali, M.; Liao, Y.; Mirjalili, R.; Klonner, M.; Sopanen, M.; Suihkonen, S.; Shen, T.; Otis, B.; Lipsanen, H. A single-pixel wireless contact lens display. J. Micromech. Microeng. 2011, 21, 125014. [Google Scholar] [CrossRef]
- De Smedt, S. Noninvasive intraocular pressure monitoring: Current insights. Clin. Ophthalmol. 2015, 9, 1385. [Google Scholar] [CrossRef] [Green Version]
- Piso, D.; Veiga-Crespo, P.; Vecino, E. Modern monitoring intraocular pressure sensing devices based on application specific integrated circuits. J. Biomater. Nanobiotechnol. 2012, 3, 18990. [Google Scholar] [CrossRef] [Green Version]
- Pandey, J.; Liao, Y.-T.; Lingley, A.; Mirjalili, R.; Parviz, B.; Otis, B.P. A fully integrated RF-powered contact lens with a single element display. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 454–461. [Google Scholar] [CrossRef]
- McDermott, M.L.; Chandler, J.W. Therapeutic uses of contact lenses. Surv. Ophthalmol. 1989, 33, 381–394. [Google Scholar] [CrossRef]
- Folch, A.; Ayon, A.; Hurtado, O.; Schmidt, M.; Toner, M. Molding of deep polydimethylsiloxane microstructures for microfluidics and biological applications. J. Biomech. Eng. 1999, 121, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Chynn, E.W.; Lopez, M.A.; Pavan-Langston, D.; Talamo, J.H. Acanthamoeba keratitis: Contact lens and noncontact lens characteristics. Ophthalmology 1995, 102, 1369–1373. [Google Scholar] [CrossRef]
- Schein, O.D.; Glynn, R.J.; Poggio, E.C.; Seddon, J.M.; Kenyon, K.R.; The Microbial Keratitis Study Group. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N. Engl. J. Med. 1989, 321, 773–778. [Google Scholar] [CrossRef]
- Everitt, D.E.; Avorn, J. Systemic effects of medications used to treat glaucoma. Ann. Intern. Med. 1990, 112, 120–125. [Google Scholar] [CrossRef]
- Kim, J.; Cha, E.; Park, J.U. Recent advances in smart contact lenses. Adv. Mater. Technol. 2020, 5, 1900728. [Google Scholar] [CrossRef]
- Savariraj, A.S.A.D.; Alam, F.; Elsherif, M.; AlQattan, B.; Khan, A.A.; Yetisen, A.K.; Butt, H. Ophthalmic Sensors and Drug Delivery. ACS Sens. 2021, 6, 2046. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, D.; de Barros, N.R.; Zare, M.R. Smart Contact Lenses for Biosensing Applications. Adv. Intell. Syst. 2021, 3, 2000263. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseinian, H.; Hosseini, S.; Martinez-Chapa, S.O.; Sher, M. A Meta-Analysis of Wearable Contact Lenses for Medical Applications: Role of Electrospun Fiber for Drug Delivery. Polymers 2022, 14, 185. https://doi.org/10.3390/polym14010185
Hosseinian H, Hosseini S, Martinez-Chapa SO, Sher M. A Meta-Analysis of Wearable Contact Lenses for Medical Applications: Role of Electrospun Fiber for Drug Delivery. Polymers. 2022; 14(1):185. https://doi.org/10.3390/polym14010185
Chicago/Turabian StyleHosseinian, Hamed, Samira Hosseini, Sergio O. Martinez-Chapa, and Mazhar Sher. 2022. "A Meta-Analysis of Wearable Contact Lenses for Medical Applications: Role of Electrospun Fiber for Drug Delivery" Polymers 14, no. 1: 185. https://doi.org/10.3390/polym14010185
APA StyleHosseinian, H., Hosseini, S., Martinez-Chapa, S. O., & Sher, M. (2022). A Meta-Analysis of Wearable Contact Lenses for Medical Applications: Role of Electrospun Fiber for Drug Delivery. Polymers, 14(1), 185. https://doi.org/10.3390/polym14010185