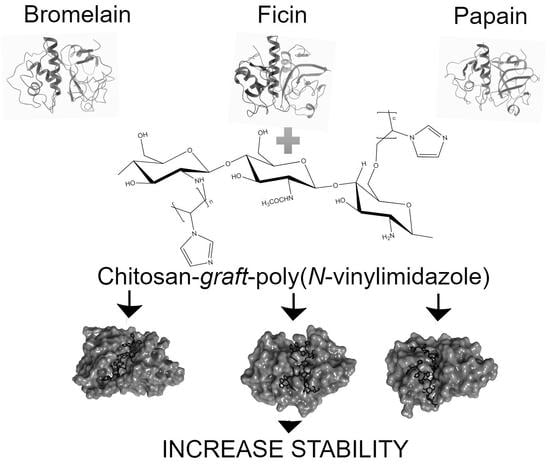
Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Cht-g-PVI Copolymers
2.3. Instrumental Section
2.4. Enzyme’s Immobilization
2.5. Molecular Docking
3. Results and Discussions
3.1. Synthesis and Characterizations of the Cht-g-PVI Copolymers
3.2. Copolymer Solution Properties
3.3. Enzyme Immobilization and Interactions with the Cht-g-PVI Copolymer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Wankhade, V. Animal-derived biopolymers in food and biomedical technology. In Biopolymer-Based Formulations, 1st ed.; Pal, K., Banerjee, I., Sarkar, P., Kim., D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier Science Publishing Co., Inc.: Amsterdam, The Netherlands, 2021; pp. 139–152. [Google Scholar] [CrossRef]
- Varma, K.; Gopi, S. Biopolymers and their role in medicinal and pharmaceutical applications. In Biopolymers and Their Industrial Applications, 1st ed.; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier Science Publishing Co., Inc.: Amsterdam, The Netherlands, 2021; pp. 175–191. [Google Scholar] [CrossRef]
- Reashmi, R.; Philip, E.; Madhavan, A.; Arun, K.B.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Gnansounou, E.; Pandey, A.; Sindhu, R. Promising eco-friendly biomaterials for future biomedicine: Cleaner production and applications of Nanocellulose. Environ. Technol. Innov. 2021, 24, 101855. [Google Scholar] [CrossRef]
- Koyyad, A.; Orsu, P. Natural gum polysaccharides as efficient tissue engineering and drug delivery biopolymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102431. [Google Scholar] [CrossRef]
- Okoro, O.V.; Amenaghawon, A.; Podstawczyk, D.; Alimoradi, H.; Khalili, M.R.; Anwar, M.; Milan, P.B.; Nie, L.; Shavandi, A. Fruit pomace-lignin as a sustainable biopolymer for biomedical applications. J. Clean. Prod. 2021, 328, 129498. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sean, T.C.; Kudaiyappan, T.; Bisuarah, U.; Miraramandi, A.; Faradjeva, A.; Abubakar, A.; Ali, A.A.; Angalene, J.L.A.A.; Kumar, S.S. Production, characterization and application of nanocarriers made of polysaccharides, proteins, bio-polyesters and other biopolymers: A review. Int. J. Biol. Macromol. 2020, 165, 3088–3105. [Google Scholar] [CrossRef]
- Berti, I.R.; Islan, G.A.; Castro, G.R. Enzymes and biopolymers. The opportunity for the smart design of molecular delivery systems. Bioresour. Technol. 2020, 322, 124546. [Google Scholar] [CrossRef]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Bierhalz, A.C.K.; Pires, A.L.R.; Moraes, A.M. Biopolymer-based films and membranes as wound dressings. In Biopolymer Membranes and Films, 1st ed.; Agostini de Moraes, M., Ferreira da Silva, C., Silveira Vieira, R., Eds.; Elsevier: San Diego, CA, USA, 2020; pp. 165–194. [Google Scholar] [CrossRef]
- Sikka, M.P.; Midha, V.K. The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Elsevier: Duxford, UK; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 463–488. [Google Scholar] [CrossRef]
- Alibolandi, M.; Bagheri, E.; Mohammadi, M.; Sameiyan, E.; Ramezani, M. Biopolymer-Based Hydrogel Wound Dressing. In Modeling and Control of Drug Delivery Systems; Azar, A.T., Ed.; Academic Press: London, UK, 2021; pp. 227–251. [Google Scholar] [CrossRef]
- Moholkar, D.N.; Sadalage, P.S.; Peixoto, D.; Paiva-Santos, A.S.; Pawar, K.D. Recent advances in biopolymer-based formulations for wound healing applications. Eur. Polym. J. 2021, 160, 110784. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohyd. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Muzzarelli, R.A.A. Chitosan for Biomaterials I; Springer: Berlin/Heidelberg, Germany, 2011; pp. 253–257. [Google Scholar]
- Holyavka, M.; Faizullin, D.; Koroleva, V.; Olshannikova, S.; Zachartchenko, N.; Zuev, Y.; Kondratyev, M.; Zakharova, E.; Artukhov, V. Novel biotechnological formulations of cysteine proteases, immobilized on chitosan. Structure, stability and activity. Int. J. Biol. Macromol. 2021, 180, 161–176. [Google Scholar] [CrossRef]
- Holyavka, M.; Pankova, S.; Koroleva, V.; Vyshkvorkina, Y.; Lukin, A.; Kondratyev, M.; Artuykhov, V. Influence of UV radiation on molecular structure and catalytic activity of free and immobilized bromelain, ficin and papain. J. Photochem. Photobiol. B Biol. 2019, 201, 111681. [Google Scholar] [CrossRef] [PubMed]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasilieva, O.S.; Akhmetova, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-biofilm and wound-healing activity of chitosan-immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizyna, E.Y.; Bogachev, M.I.; Artuykhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical Properties and Anti-Biofilm Activity of Chitosan-Immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef]
- Sorokin, A.; Lavlinskaya, M. Synthesis of the superabsorbents enriched in chitosan derivatives with excellent water absorption properties. Polym. Bull. 2022, 79, 407–427. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Kuznetsov, V.A.; Lavlinskaya, M.S. Synthesis of graft copolymers of carboxymethyl cellulose and N,N-dimethylaminoethyl methacrylate and their study as Paclitaxel carriers. Polym. Bull. 2021, 78, 2975–2992. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Sorokin, A.V.; Lavlinskaya, M.S.; Sinelnikov, A.A.; Bykovskiy, D.V. Graft copolymers of carboxymethyl cellulose with N-vinylimidazole: Synthesis and application for drug delivery. Polym. Bull. 2019, 76, 4929–4949. [Google Scholar] [CrossRef]
- Dağaş, D.E.; Danelyan, G.V.; Ghaffarlou, M.; Zezina, E.A.; Abramchuk, S.S.; Feldman, V.I.; Olgun, G.; Zezin, A.A. Generation of spatially ordered metal–polymer nanostructures in the irradiated dispersions of poly(acrylic acid)–poly(vinylimidazole)—Cu2+ complexes. Colloid Polym. Sci. 2020, 298, 193–202. [Google Scholar] [CrossRef]
- Genç, F.; Uzun, C.; Güven, O. Quaternized poly(1-vinylimidazole) hydrogel for anion adsorption. Polym. Bull. 2015, 73, 179–190. [Google Scholar] [CrossRef]
- Kodama, H.; Miyajima, T.; Tabuchi, H.; Ishiguro, S. A calorimetric study of the acid dissociation of the conjugate acids of poly(N-vinylimidazole) and polyallylamine. Colloid Polym. Sci. 2020, 278, 1–7. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Simenel, I.A.; Kulakova, V.K.; Kurskaya, E.A.; Babushkina, T.A.; Klimova, T.P.; Burova, T.V.; Dubovik, A.S.; Galaev, I.Y.; Mattiason, B.; et al. Synthesis and Studies of N-Vinylcaprolactam/N-Vinylimidazole Copolymers that Exhibit the “Proteinlike” Behavior in Aqueous Media. Macromolecules 2003, 36, 7308–7323. [Google Scholar] [CrossRef]
- Brix, K.; Stöcker, W. Proteases: Structure and Function; Springer: Wien, Austria, 2013. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Faar, A.L.; Randal, R.J. Protein measurement with folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sabirova, A.R.; Rudakova, N.L.; Balaban, N.P.; Ilyinskaya, O.N.; Demidyuk, I.V.; Kostrov, S.V.; Rudenskaya, G.N.; Sharipova, M.R. A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett. 2010, 584, 4419–4425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.; Misra, B. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Maldas, D. Graft copolymerization onto cellulosics. Prog. Polym. Sci. 1984, 10, 171–270. [Google Scholar] [CrossRef]
- Bamford, C.H.; Schofield, E. Non-classical free-radical polymerization: Degradative addition to monomer in the polymerization of 1-vinylimidazole. Polymer 1981, 22, 1227–1235. [Google Scholar] [CrossRef]
- Ebdon, J.R.; Huckerby, T.N.; Hunter, T.C. Free-radical aqueous slurry polymerizations of acrylonitrile: 1. End-groups and other minor structures in polyacrylonitriles initiated by ammonium persulfate/sodium metabisulfite. Polymer 1994, 35, 250–256. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Microwave assisted synthesis of poly (N-vinylimidazole) grafted chitosan as an effective adsorbent for mercury (II) removal from aqueous solution: Equilibrium, kinetic, thermodynamics and regeneration studies. J. Dispers. Sci. Technol. 2020, 41, 828–840. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Lavlinskaya, M.S.; Ostankova, I.V.; Shatalov, G.V.; Shikhaliev, K.S.; Ryzhkova, E.A. Synthesis of N-vinylformamide and 1-vinyl-(1-methacryloyl)-3,5-dimethylpyrazole copolymers and their extraction ability in relation to histidine in water-salt media. Polym. Bull. 2018, 75, 1237–1251. [Google Scholar] [CrossRef]
- Buller, A.R.; Townsend, C.A. Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad. Proc. Natl. Acad. Sci. USA 2013, 110, E653–E661. [Google Scholar] [CrossRef] [Green Version]







| No | Copolymer Name | Cht:VI, mol | VI, mL |
|---|---|---|---|
| 1 | Cht-g-PVI-3 | 1:3 | 0.62 |
| 2 | Cht-g-PVI-5 | 1:5 | 1.05 |
| 3 | Cht-g-PVI-10 | 1:10 | 2.10 |
| 4 | Cht-g-PVI-20 | 1:20 | 4.12 |
| No | Copolymer Name | Yield, % | VI in the Copolymer, % mol | GE, % | FG × 102 | Mη of Grafted PVI |
|---|---|---|---|---|---|---|
| 1 | Cht-g-PVI-3 | 67 | 31 | 40 | 0.76 | 9436 |
| 2 | Cht-g-PVI-5 | 59 | 49 | 38 | 1.31 | 11,769 |
| 3 | Cht-g-PVI-10 | 40 | 63 | 25 | 1.74 | 15,641 |
| 4 | Cht-g-PVI-20 | 34 | 66 | 19 | 1.77 | 17,583 |
| No | Copolymer Name | Dh, nm | c*, % w/v | ζ, mV | Mobility, µmcm/Vs | Conductivity, mS/cm |
|---|---|---|---|---|---|---|
| 1 | Cht-g-PVI-3 | 675 ± 16 | 0.0023 | 44.0 ± 1.6 | 3.45 ± 0.13 | 1.32 ± 0.04 |
| 2 | Cht-g-PVI-5 | 765 ± 19 | 0.0120 | 47.5 ± 1.2 | 3.73 ± 0.09 | 1.79 ± 0.07 |
| 3 | Cht-g-PVI-10 | 995 ± 23 | 0.0240 | 54.4 ± 2.2 | 4.26 ± 0.18 | 2.45 ± 0.12 |
| 4 | Cht-g-PVI-20 | 1449 ± 39 | 0.0260 | 55.3 ± 3.1 | 4.33 ± 0.25 | 3.36 ± 0.23 |
| Polymer | Bromelain | Ficin | Papain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Enzyme Content, mg × g−1 | Proteolytic Activity, | Enzyme Content, mg × g−1 | Proteolytic Activity, | Enzyme Content, mg × g−1 | Proteolytic Activity, | ||||
| U × mL−1 | % | U × mL−1 | % | U × mL−1 | % | ||||
| Native enzyme | - | 97 ± 7 | 100 | - | 96 ± 2 | 100 | - | 95 ± 4 | 100 |
| Cht-g-PVI-3 | 33.6 ± 1 | 78 ± 2 | 80 | 14.9 ± 1 | 39 ± 8 | 41 | 30.9 ± 3 | 97 ± 1 | 100 |
| Cht 350 kDa | 18.6 | 115 ± 10 | 118 | 2.6 | 88 ± 8 | 92 | 7.2 | 94 ± 8 | 100 |
| Affinity, kcal/mol | Amino Acid Residues Forming | |
|---|---|---|
| H-Bonds, Length, Å | Other Interactions | |
| Bromelain amino acids interacting with Cht-g-PVI | ||
| −8.3 | Gln20, 3.29 Asn21, 2.92 Lys64, 3.19 Ala136, 2.94 Gln141, 2.94 Lys179, 3.09 | Val14, Val17, Lys18, Asn19, Pro22, Cys23, Gly24, Cys26, Phe29, Ala33, Gly65, Gly66, Ala133, Asn137, Phe140, Leu156, Asn157, His158, Ala159, Val160, Thr161, Ile163, Ala178, Trp180, Gly184, Tyr185, Ile186 |
| Ficin amino acids interacting with Cht-g-PVI | ||
| −6.2 | Asp14, 3.04 Ile16, 2.56 Arg17, 2.86 Leu45, 2.8 Ser47, 2.84 Glu86, 2.89 Thr92, 3.2 Arg94, 3.0 | Arg8, Val13, Pro15, Asp18, Gly20, Lys21, Pro46, Tyr91, Ala93, Gly185, Thr186, Lys187, Glu191, Tyr193 |
| Papain amino acids interacting with Cht-g-PVI | ||
| −6.8 | Cys25, 2.71 His159, 3.1 | Gln19, Gly20, Gly23, Tyr61, Asn64, Gly65, Gly66, Tyr67, Pro68, Val133, Gln135, Ala137, Gly138, Gln142, Asn155, Lys156, Val157, Asp158, Trp177, Trp181, Thr204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokin, A.V.; Olshannikova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Zuev, Y.F.; Artukhov, V.G. Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain. Polymers 2022, 14, 2279. https://doi.org/10.3390/polym14112279
Sorokin AV, Olshannikova SS, Lavlinskaya MS, Holyavka MG, Faizullin DA, Zuev YF, Artukhov VG. Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain. Polymers. 2022; 14(11):2279. https://doi.org/10.3390/polym14112279
Chicago/Turabian StyleSorokin, Andrey V., Svetlana S. Olshannikova, Maria S. Lavlinskaya, Marina G. Holyavka, Dzhigangir A. Faizullin, Yuriy F. Zuev, and Valeriy G. Artukhov. 2022. "Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain" Polymers 14, no. 11: 2279. https://doi.org/10.3390/polym14112279
APA StyleSorokin, A. V., Olshannikova, S. S., Lavlinskaya, M. S., Holyavka, M. G., Faizullin, D. A., Zuev, Y. F., & Artukhov, V. G. (2022). Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain. Polymers, 14(11), 2279. https://doi.org/10.3390/polym14112279