Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photolithography
2.2. Replica of a Nepenthes Peristome-like Structure by PDMS
2.3. MPC Immobilization by Atmospheric Plasma
2.4. Anti-Bacterial Adhesion Capability
2.5. Anti-Protein Adhesion Capability
2.6. Cell Attachment Tests
2.7. Biocompatibility
2.8. One-Way Liquid Transfer Capability
2.9. Characterizations
3. Results and Discussion
3.1. Optical Microscope (OM) Analysis
3.2. Scanning Electron Microscope (SEM) Observation
3.3. Hydrophilicity by Contact Angle Measurements
3.4. FTIR Spectrum Analysis
3.5. XPS Spectrum Analysis
3.6. Antibacterial Adhesion Test
3.7. Anti-Protein Adhesion Test
3.8. Cells Attachment Test
3.9. Biocompatibility Test
3.10. One-Way Liquid Transfer Capability
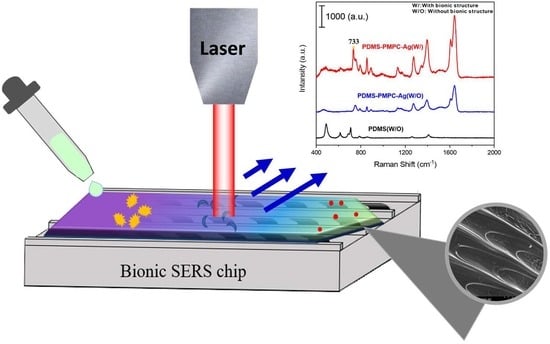
3.11. SERS Detection by Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, L.; Chen, H.; Dong, Z.; Zhang, D. Surfaces inspired by the Nepenthes peristome for unidirectional liquid transport. Adv. Mater. 2017, 29, 1702995. [Google Scholar] [CrossRef] [PubMed]
- Bico, J.; Marzolin, C.; Quéré, D. Pearl drops. EPL 1999, 47, 220. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Z.; Qiu, Z.; Feng, S.; Xie, Y.; Leng, D.; Tian, X. A brief review of bio-inspired surface technology and application toward underwater drag reduction. Ocean Eng. 2020, 199, 106962. [Google Scholar] [CrossRef]
- Russell, T. Surface-responsive materials. Science 2002, 297, 964–967. [Google Scholar] [CrossRef]
- Willermet, C. Biological Anthropology in 2015: Open access, biocultural interactions, and social change. Am. Anthropol. 2016, 118, 317–329. [Google Scholar] [CrossRef]
- Lan, J.; Han, J. Research on the radiation characteristics of aerodynamic noises of a simplified bogie of the high-speed train. J. Vibroeng. 2017, 19, 2280–2293. [Google Scholar]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Vincent, J. Structural biomaterials. In Structural Biomaterials; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- Fayemi, P.-E.; Maranzana, N.; Aoussat, A.; Bersano, G. Bio-inspired design characterisation and its links with problem solving tools. In Proceedings of the DS 77: Proceedings of the DESIGN 2014 13th International Design Conference, Dubrovnik, Croatia, 19–22 May 2014; pp. 173–182. [Google Scholar]
- Zhang, P.; Chen, H.; Li, L.; Liu, H.; Liu, G.; Zhang, L.; Zhang, D.; Jiang, L. Bioinspired smart peristome surface for temperature-controlled unidirectional water spreading. ACS Appl. Mater. Interfaces 2017, 9, 5645–5652. [Google Scholar] [CrossRef]
- Gestrelius, S.; Lyngstadaas, S.; Hammarström, L. Emdogain–periodontal regeneration based on biomimicry. Clin. Oral Investig. 2000, 4, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, W.; Su, B.-L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Herminghaus, S. Roughness-induced non-wetting. EPL 2007, 79, 59901. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Superhydrophobic Surfaces and Emerging Applications: Non-Adhesion. Energy Green Eng. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, K.; Bai, Q.; Chen, J.; Wang, B. Modeling and experimental analysis of microburr formation considering tool edge radius and tool-tip breakage in microend milling. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Processing Meas. Phenom. 2009, 27, 1531–1535. [Google Scholar] [CrossRef]
- Chen, T.L.; Lin, Y.P.; Chien, C.H.; Chen, Y.C.; Yang, Y.J.; Wang, W.L.; Chien, L.F.; Hsueh, H.Y. Fabrication of Frog-Skin-Inspired Slippery Antibiofouling Coatings Through Degradable Block Copolymer Wrinkling. Adv. Funct. Mater. 2021, 31, 2104173. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Gu, J.; Fan, T.; Liu, Q.; Su, H.; Zhu, S. Inspiration from butterfly and moth wing scales: Characterization, modeling, and fabrication. Prog. Mater. Sci. 2015, 68, 67–96. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Hsiao, C.-W.; Lin, C.-H.; Huang, L.-Y.; Chen, J.-S.; Yang, M.-C.; Liu, T.-Y. Bionic 3D periodic nanostructures by Ag nano-islands deposited on cicada wings for rapid SERS detection. Surf. Coat. Technol. 2022, 436, 128323. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Wang, J.; Shi, Z.; Du, L. Reproducible Flexible SERS Substrates Inspired by Bionic Micro-Nano Hierarchical Structures of Rose Petals. Adv. Mater. Interfaces 2022, 9, 2102468. [Google Scholar] [CrossRef]
- Li, C.; Wu, L.; Yu, C.; Dong, Z.; Jiang, L. Peristome-Mimetic Curved Surface for Spontaneous and Directional Separation of Micro Water-in-Oil Drops. Angew. Chem. 2017, 129, 13811–13816. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, L.; Ru, Y.; Li, N.; Li, C.; Gao, C.; Dong, Z.; Jiang, L. Drop cargo transfer via unidirectional lubricant spreading on peristome-mimetic surface. ACS Nano 2018, 12, 11307–11315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, D.; Wang, D.; Zhang, L.; Feng, L.; Zhang, X. A bioinspired flexible film fabricated by surface-tension-assisted replica molding for dynamic control of unidirectional liquid spreading. ACS Appl. Mater. Interfaces 2019, 11, 48505–48511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yu, C.; Li, C.; Jiang, L.; Dong, Z. Droplets Crawling on Peristome-Mimetic Surfaces. Adv. Funct. Mater. 2020, 30, 1908066. [Google Scholar] [CrossRef]
- He, X.; Ge, C.; Zheng, X.; Tang, B.; Chen, L.; Li, S.; Wang, L.; Zhang, L.; Xu, Y. Rapid identification of alpha-fetoprotein in serum by a microfluidic SERS chip integrated with Ag/Au Nanocomposites. Sens. Actuators B Chem. 2020, 317, 128196. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Zhao, H.; Li, S.; Chen, L. Design and preparation of centrifugal microfluidic chip integrated with SERS detection for rapid diagnostics. Talanta 2019, 194, 903–909. [Google Scholar] [CrossRef]
- Feng, W.; Brash, J.L.; Zhu, S. Non-biofouling materials prepared by atom transfer radical polymerization grafting of 2-methacryloloxyethyl phosphorylcholine: Separate effects of graft density and chain length on protein repulsion. Biomaterials 2006, 27, 847–855. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S.; Cheng, G.; Vaisocherova, H.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Film thickness dependence of protein adsorption from blood serum and plasma onto poly (sulfobetaine)-grafted surfaces. Langmuir 2008, 24, 9211–9214. [Google Scholar] [CrossRef]
- Chen, J.-S.; Liu, T.-Y.; Tsou, H.-M.; Ting, Y.-S.; Tseng, Y.-Q.; Wang, C.-H. Biopolymer brushes grown on PDMS contact lenses by in situ atmospheric plasma-induced polymerization. J. Polym. Res. 2017, 24, 69. [Google Scholar] [CrossRef]
- Chen, J.-S.; Ting, Y.-S.; Tsou, H.-M.; Liu, T.-Y. Highly hydrophilic and antibiofouling surface of zwitterionic polymer immobilized on polydimethylsiloxane by initiator-free atmospheric plasma-induced polymerization. Surf. Coat. Technol. 2018, 344, 621–625. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Ting, Y.-S.; Chen, B.-Y.; Cheng, Y.-W.; Liu, T.-Y. Bionic shark skin replica and zwitterionic polymer brushes functionalized PDMS membrane for anti-fouling and wound dressing applications. Surf. Coat. Technol. 2020, 391, 125663. [Google Scholar] [CrossRef]
- Ito, H.; Onitsuka, S.; Gappa, R.; Saitoh, H.; Roacho, R.; Pannell, K.; Suzuki, T.; Niibe, M.; Kanda, K. Fabrication of amorphous silicon carbide films from decomposition of tetramethylsilane using ECR plasma of Ar. Proc. J. Phys. Conf. Ser. 2013, 441, 012039. [Google Scholar] [CrossRef]
- Hoek, I.; Tho, F.; Arnold, W.M. Sodium hydroxide treatment of PDMS based microfluidic devices. Lab Chip 2010, 10, 2283–2285. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, J.; Ge, Y.; Zhang, X.; Shi, H.; Yang, K.; Gao, X.; Shi, S.; Gong, Y. Fabrication of stable biomimetic coating on PDMS surface: Cooperativity of multivalent interactions. Appl. Surf. Sci. 2019, 469, 720–730. [Google Scholar] [CrossRef]
- D’Angelo, A.J.; Panzer, M.J. Decoupling the ionic conductivity and elastic modulus of gel electrolytes: Fully zwitterionic copolymer scaffolds in lithium salt/ionic liquid solutions. Adv. Energy Mater. 2018, 8, 1801646. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Wu, C.-H.; Syu, W.-L.; Ho, P.-C.; Tseng, Z.-L.; Yang, M.-C.; Lin, C.-C.; Chen, C.-C.; Chen, C.-C.; Liu, T.-Y. Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection. Polymers 2022, 14, 2465. https://doi.org/10.3390/polym14122465
Lin Y-T, Wu C-H, Syu W-L, Ho P-C, Tseng Z-L, Yang M-C, Lin C-C, Chen C-C, Chen C-C, Liu T-Y. Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection. Polymers. 2022; 14(12):2465. https://doi.org/10.3390/polym14122465
Chicago/Turabian StyleLin, Yen-Ting, Chun-Hao Wu, Wei-Lin Syu, Po-Cheng Ho, Zi-Ling Tseng, Ming-Chien Yang, Chin-Ching Lin, Cheng-Chen Chen, Cheng-Cheung Chen, and Ting-Yu Liu. 2022. "Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection" Polymers 14, no. 12: 2465. https://doi.org/10.3390/polym14122465
APA StyleLin, Y. -T., Wu, C. -H., Syu, W. -L., Ho, P. -C., Tseng, Z. -L., Yang, M. -C., Lin, C. -C., Chen, C. -C., Chen, C. -C., & Liu, T. -Y. (2022). Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection. Polymers, 14(12), 2465. https://doi.org/10.3390/polym14122465