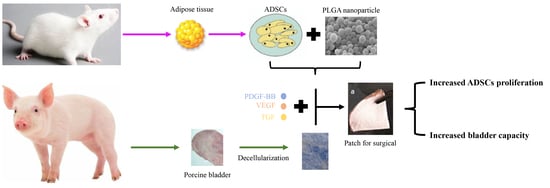
Construction of Tissue-Engineered Bladder Scaffolds with Composite Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Methods
2.2.1. Adipose-Derived Stem Cells (ADSCs) Culture
2.2.2. PLGA-CS and PLGA-SA Cross-Linked Microparticle Emulsion Preparation
2.2.3. Bladder Acellular Matrix (BAM) Preparation
2.2.4. Preparation of Surgical Patch
2.2.5. Transplantation Experiment in Rats In Vivo
2.2.6. Bladder Capacity Maximum (BCM) Test
2.2.7. Histological and Immunohistochemical Analyses of the Cell Growth in Patch
3. Results and Discussion
3.1. Characterisation of PLGA-Microparticles
3.2. Prepared Bladder Acellular Matrix (BAM) and Surgical Patch
3.3. Effect of Surgical Patches Containing PLGA Microparticles on ADSCs
3.4. Histological and Immunohistochemical Analyses of Cell Growth in Patches
3.5. Bladder Capacity of the Reconstructed Bladder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering approaches for bladder regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.J.; Olson, J.; Atala, A.; Kim, B. Regenerative medicine strategies for treating neurogenic bladder. Int. Neurourol. J. 2011, 15, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Zarrintaj, P.; Ofmati, F.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdas, F.; Naji, M.; Sarhangnejad, R.; Rajabi-Zeleti, S.; Mirzadeh, H.; Zandi, M.; Saeed, M. Comparing supportive properties of poly lactic-co-glycolic acid (PLGA), PLGA/collagen and human amniotic membrane for human urothelial and smooth muscle cells engineering. Urol. J. 2014, 11, 1620–1628. [Google Scholar]
- Salem, S.A.; Rashidbenam, Z.; Jasman, M.H.; Ho, C.C.H.; Sagap, I.; Singh, R.; Yusof, M.R.; Zainuddin, Z.M.; Idrus, R.B.H.; Ng, M.H. Incorporation of smooth muscle cells derived from human adipose stem cells on poly (lactic-co-glycolic acid) scaffold for the reconstruction of subtotally resected urinary bladder in athymic rats. Tissue Eng. Regen. Med. 2020, 17, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for tissue engineering. Adv. Exp. Med. Biol. 2018, 1077, 475–485. [Google Scholar]
- Follin, B.; Juhl, M.; Cohen, S.; Pederson, A.E.; Gad, M.; Kastrup, J.; Ekblond, A. Human adipose- derived stromal cells in a clinically applicable injectable alginate hydrogel: Phenotypic and immunomodulatory evaluation. Cytotherapy 2015, 17, 1104–1118. [Google Scholar] [CrossRef]
- Wang, Q.; Jamal, S.; Detamore, M.S.; Berkland, C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J. Biomed. Mater. Res. A 2011, 96, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Pandini, F.E.; Kubo, F.M.M.; Plepis, A.M.G.; Martins, V.D.C.A.; da Cunha, M.R.; Silva, V.R.; Hirota, V.B.; Lopes, E.; Menezes, M.A.; Pelegrine, A.A.; et al. In vivo study of nasal bone reconstruction with collagen, elastin and chitosan membranes in abstainer and alcoholic rats. Polymers 2022, 14, 188. [Google Scholar] [CrossRef]
- Bulut, E. Development and optimization of Fe3+ crosslinked sodium alginate-methylcellulose semi-interpenetrating polymer network beads for controlled release of ibuprofen. Int. J. Biol. Macromol. 2021, 168, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; He, J.; Sang, F.; Wang, Q.; Chen, L.; Cui, S.; Ding, B. Enhanced bone formation in electrospun poly (L-lactic-co-glycolic acid)-tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.K. Biocompatible polymers and their potential biomedical applications: A review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef]
- Haider, A.; Versace, D.-L.; Gupta, K.C.; Kang, I.-K. Pamidronic acid-grafted nHA/PLGA hybrid nanofiber scaffolds suppress osteoclastic cell viability and enhance osteoblastic cell activity. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 7596–7604. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Gubanska, I.; Drewa, G.; Drewa, T. Application of bladder acellular matrix in urinary bladder regeneration: The state of the art and future directions. Biomed. Res. Int. 2015, 2015, 613439. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, L.; Xu, Z.; Ping, W.; Liu, J.; Zhou, C.; Wang, M.; Ruipeng, J. Hypoxia-preconditioned adipose-derived endothelial progenitor cells promote bladder augmentation. Tissue Eng. Part A 2020, 26, 78–92. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Idrus, R.B.H. Collagen type I: A versatile biomaterial. Adv. Exp. Med. Biol. 2018, 1077, 389–414. [Google Scholar]
- Chua, M.E.; Farhat, W.A.; Ming, J.M.; McCammon, K.A. Review of clinical experience on biomaterials and tissue engineering of urinary bladder. World J. Urol. 2020, 38, 2081–2093. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Jundzill, A.; Rasmus, M.; Adamowicz, J.; Balcerczyk, D.; Buhl, M.; Warda, K.; Buchholz, L.; Gagat, M.; Grzanka, D.; et al. Understanding the role of mesenchymal stem cells in urinary bladder regeneration-a preclinical study on a porcine model. Stem Cell Res. Ther. 2018, 9, 328. [Google Scholar] [CrossRef]
- Pearson, R.G.; Bhandari, R.; Quirk, R.A.; Shakesheff, K.M. Recent advances in tissue engineering. J. Long Term Effects Med. Implants 2017, 27, 199–231. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Lim, G.J.; Kwon, T.G.; Chun, S.Y.; Lim, G.J.; Kwon, T.G.; Kwak, E.K.; Kim, B.W.; Atala, A.; Yoo, J.J. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials 2007, 28, 4251–4256. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Appelt-Menzel, A.; Kurdyn, S.; Walles, H.; Groeber, F. Generation of a three-dimensional full thickness skin equivalent and automated wounding. J. Vis. Exp. 2015, 96, 52576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Feng, C.; Liu, Y.; Peng, X.; Chen, S.; Xiao, D.; Wang, H.; Li, Z.; Xu, Y.; Lu, M. Erratum: A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction. Theranostics 2018, 8, 3153–3163. [Google Scholar] [CrossRef] [Green Version]
- Daša, Z.; Poljšak, K.M.; Kreft, M.E. Co-culturing porcine normal urothelial cells, urinary bladder fibroblasts and smooth muscle cells for tissue engineering research. Cell Biol. Int. 2018, 42, 411–424. [Google Scholar]
- Song, H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell. 2018, 22, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: Beyond crating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, D.; Chen, Y.; Kong, Q.; Zhang, Q.; Liu, C.; Tian, Y.; Fan, C.; Meng, L.; Zhu, H.; et al. Ureter tissue engineering with vessel extracellular matrix and differentiated urine-derived stem cells. Acta Biomater. 2019, 88, 266–279. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Qi, N.; Guo, T.; Wang, C.; Huang, Z.; Du, Z.; Xu, D.; Zhao, Y.; Tian, H. Construction of Tissue-Engineered Bladder Scaffolds with Composite Biomaterials. Polymers 2022, 14, 2654. https://doi.org/10.3390/polym14132654
Li W, Qi N, Guo T, Wang C, Huang Z, Du Z, Xu D, Zhao Y, Tian H. Construction of Tissue-Engineered Bladder Scaffolds with Composite Biomaterials. Polymers. 2022; 14(13):2654. https://doi.org/10.3390/polym14132654
Chicago/Turabian StyleLi, Wenjiao, Na Qi, Tingting Guo, Chao Wang, Ziwei Huang, Zhouyuan Du, Dingwen Xu, Yin Zhao, and Hong Tian. 2022. "Construction of Tissue-Engineered Bladder Scaffolds with Composite Biomaterials" Polymers 14, no. 13: 2654. https://doi.org/10.3390/polym14132654
APA StyleLi, W., Qi, N., Guo, T., Wang, C., Huang, Z., Du, Z., Xu, D., Zhao, Y., & Tian, H. (2022). Construction of Tissue-Engineered Bladder Scaffolds with Composite Biomaterials. Polymers, 14(13), 2654. https://doi.org/10.3390/polym14132654