A New Approach Utilizing Aza-Michael Addition for Hydrolysis-Resistance Non-Ionic Waterborne Polyester
Abstract
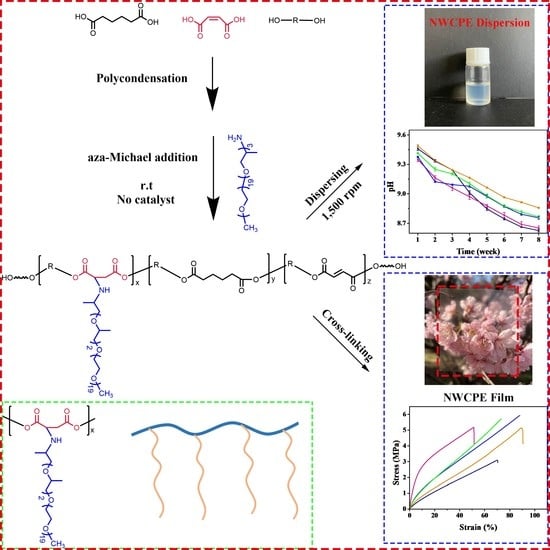
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Linear Polyesters
2.2.2. Synthesis of Comb-like Polyester
2.2.3. Preparation of Non-Ionic Waterborne Comb-like Polyester (NWCPE) Dispersion
2.2.4. Cross-Linking of the NWCPE Dispersions
2.3. Characterizations
3. Results and Discussion
3.1. Structure Characterizations
3.2. The Influence of Various Factors on Polyester and Polyester Dispersion
3.2.1. Effect of the Catalyst Type
3.2.2. Effect of the Reaction Temperature
3.2.3. The Influence of MA/HA Monomer Ratio
3.2.4. The Influence of the Rigid Monomer Content
3.3. Thermogravimetric Analysis
3.4. Tensile Properties of the Cross-Linked NWCPE Films
3.5. The Morphology and Light Transmittance of the Cured Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, S.; Inglefield, D.L.; Velev, O.D. Revisiting the colloidal fundamentals of water-dispersible polyesters: Interactions and self-assembly of polymer nanoaggregates in water. Soft Matter 2018, 14, 2118–2130. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Guan, T.; Hou, C.; Wu, J.; Wang, G.; Ji, X.; Wang, B. Preparation, properties and application of waterborne hydroxyl-functional polyurethane/acrylic emulsions in two-component coatings. J. Coat. Technol. Res. 2015, 12, 505–512. [Google Scholar] [CrossRef]
- Geeti, D.K.; Niranjan, K. Environmentally benign bio-based waterborne polyesters: Synthesis, thermal- and bio-degradation studies. Prog. Org. Coat. 2019, 127, 419–428. [Google Scholar] [CrossRef]
- Muto, A.; Toshin, K.; Shibao, F.; Ueda, K. Effect of melamine enrichment on the structure of water-based polyester/melamine films and properties of pre-painted steel sheets. Prog. Org. Coat. 2021, 150, 105963. [Google Scholar] [CrossRef]
- Wei, L.; Yue, G.; Hongjian, P.; Hongying, C. Dispersion stability of titanium dioxide in aqueous isopropanol with polymer dispersant. J. Coat. Technol. Res. 2020, 17, 1083–1090. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. WIREs. Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef]
- Pierlot, C.; Ontiveros, J.F.; Royer, M.; Catté, M.; Salager, J.-L. Emulsification of viscous alkyd resin by catastrophic phase inversion with nonionic surfactant. Colloid Surf. A 2018, 536, 113–124. [Google Scholar] [CrossRef]
- Ma, S.; Qian, J.; Zhuang, Q.; Li, X.; Kou, W.; Peng, S. Synthesis and application of water-soluble hyperbranched polyester modified by trimellitic anhydride. J. Macromol. Sci. A. 2018, 55, 414–421. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wu, X.L.; Hao, T.H.; Hu, G.H.; Jiang, T.; Zhang, Q.C.; Zhao, H. Structure design, fabrication and property investigation of water-based polyesters with notable surface hydrophilicity. New J. Chem. 2018, 42, 20015–20023. [Google Scholar] [CrossRef]
- Torres G, M.Y.; Bravo L, M.K.; Flórez M, S.A.; Simon, P.; Aguirre P, J.J.; Macías L, M.A.; Gauthier, G.H. Study of applicability in an aqueous paint of the blue pigment YIn0.95Mn0.05O3. Dye. Pigment. 2018, 156, 17–25. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Yuan, Q.X.; Gong, S.L. Preparation and characterization of self-emulsifying poly(ethylene glycol) methyl ether methacrylate grafted polyacrylate copolymers modified by waterborne polyester. J. Appl. Polym. Sci. 2021, 139, e51988. [Google Scholar] [CrossRef]
- Alemdar, N.; Erciyes, A.T.; Bicak, N. Preparation of unsaturated polyesters using boric acid as mild catalyst and their sulfonated derivatives as new family of degradable polymer surfactants. Polymer 2010, 51, 5044–5050. [Google Scholar] [CrossRef]
- Rokicki, G.; Wodzicki, H. Waterborne unsaturated polyester resins. Macromol. Mater. Eng. 2000, 278, 17–22. [Google Scholar] [CrossRef]
- Hazarika, D.; Karak, N. Waterborne Sustainable Tough Hyperbranched Aliphatic Polyester Thermosets. ACS Sustain. Chem. Eng. 2015, 3, 2458–2468. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, P.; Cheng, F.; Zhou, M.; Zhang, K.; Wang, T. Preparation of waterborne elastic polyesters by chain extension with isophorone diisocyanate as a chain extender. J. Appl. Polym. Sci. 2019, 137, 48453. [Google Scholar] [CrossRef]
- Hsiao, K.J.; Kuo, J.L.; Tang, J.W.; Chen, L.T. Physics and kinetics of alkaline hydrolysis of cationic dyeable poly(ethylene terephthalate) (CDPET) and polyethylene glycol (PEG)–modified CDPET polymers: Effects of dimethyl 5-sulfoisophthalate sodium salt/PEG content and the number-average molecular weight of the PEG. J. Appl. Polym. Sci. 2005, 98, 550–556. [Google Scholar] [CrossRef]
- Tsai, H.C.; Hong, P.D.; Yen, M.S. Preparation and physical properties of nonionic aqueous polyurethane coatings containing different side chain PEGME length. J. Appl. Polym. Sci. 2008, 108, 2266–2273. [Google Scholar] [CrossRef]
- Nabiyan, A.; Max, J.B.; Schacher, F.H. Double hydrophilic copolymers-synthetic approaches, architectural variety, and current application fields. Chem. Soc. Rev. 2022, 51, 995–1044. [Google Scholar] [CrossRef]
- Mehravar, E.; Leiza, J.R.; Asua, J.M. Performance of latexes containing nano-sized crystalline domains formed by comb-like polymers. Polymer 2016, 96, 121–129. [Google Scholar] [CrossRef]
- Li, B.; Peng, D.; Zhao, N.; Mu, Q.; Li, J. The physical properties of nonionic waterborne polyurethane with a polyether as side chain. J. Appl. Polym. Sci. 2013, 127, 1848–1852. [Google Scholar] [CrossRef]
- Taniguchi, I.; Mayes, A.M.; Chan, E.W.L.; Griffith, L.G. A Chemoselective Approach to Grafting Biodegradable Polyesters. Macromolecules 2005, 38, 216–219. [Google Scholar] [CrossRef]
- Newman, M.S. Some Observations Concerning Steric Factors. J. Am. Chem. Soc. 1950, 72, 4783–4786. [Google Scholar] [CrossRef]
- Riva, R.; Schmeits, S.; Jérôme, C.; Jérôme, R.; Lecomte, P. Combination of Ring-Opening Polymerization and “Click Chemistry”: Toward Functionalization and Grafting of Poly(ε-caprolactone). Macromolecules 2007, 40, 796–803. [Google Scholar] [CrossRef]
- Genest, A.; Portinha, D.; Fleury, E.; Ganachaud, F. The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro)molecules and materials. Prog. Polym. Sci. 2017, 72, 61–110. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef] [Green Version]
- Rulev, A.Y. Aza-Michael reaction: Achievements and prospects. Russ. Chem. Rev. 2011, 80, 197–218. [Google Scholar] [CrossRef]
- Noordzij, G.J.; Wilsens, C. Cascade aza-Michael Addition-Cyclizations; Toward Renewable and Multifunctional Carboxylic Acids for Melt-Polycondensation. Front. Chem. 2019, 7, 729. [Google Scholar] [CrossRef]
- Bosica, G.; Debono, A.J. Uncatalyzed, green aza-Michael addition of amines to dimethyl maleate. Tetrahedron 2014, 70, 6607–6612. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, Z.; Leng, X.; Li, Y. Facile preparation of stereochemistry-controllable biobased poly(butylene maleate-co-butylene fumarate) unsaturated copolyesters: A chemoselective polymer platform for versatile functionalization via aza-Michael addition. Polym. Chem-UK 2018, 9, 5426–5441. [Google Scholar] [CrossRef]
- Li, R.Q.; Huang, T.; Gong, S.L. Preparation of high hydroxyl self-emulsifying polyester and compounding with acrylate. J. Appl. Polym. Sci. 2019, 137, 48278. [Google Scholar] [CrossRef]
- Grobelny, J.N.m.r. study of maleate (cis)—fumarate (trans) isomerism in unsaturated polyesters and related compounds. Polymer 1995, 36, 4215–4222. [Google Scholar] [CrossRef]
- Tang, T.; Takasu, A. Facile synthesis of unsaturated polyester-based double-network gels via chemoselective cross-linking using Michael addition and subsequent UV-initiated radical polymerization. RSC Adv. 2015, 5, 819–829. [Google Scholar] [CrossRef]
- Farmer, T.J.; Clark, J.H.; Macquarrie, D.J.; Ogunjobi, J.K.; Castle, R.L. Post-polymerisation modification of bio-derived unsaturated polyester resins via Michael additions of 1,3-dicarbonyls. Polym. Chem. 2016, 7, 1650–1658. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, J.; Salmi, T.; Immonen, K.; Paatero, E.; Nyholm, P. Kinetic Model for the Homogeneously Catalyzed Polyesterification of Dicarboxylic Acids with Diols. Ind. Eng. Chem. Res. 1996, 35, 3951–3963. [Google Scholar] [CrossRef]
- Kang, H.; Li, M.; Tang, Z.; Xue, J.; Hu, X.; Zhang, L.; Guo, B. Synthesis and characterization of biobased isosorbide-containing copolyesters as shape memory polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 7877–7886. [Google Scholar] [CrossRef]
- DeRosa, C.A.; Kua, X.Q.; Bates, F.S.; Hillmyer, M.A. Step-Growth Polyesters with Biobased (R)-1,3-Butanediol. Ind. Eng. Chem. Res. 2020, 59, 15598–15613. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Tsanaktsis, V.; Nikolaidis, N.; Kostoglou, M.; Papageorgiou, G.Z.; Lambropoulou, D.A.; Bikiaris, D.N. Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation. Polym. Chem. 2017, 8, 6895–6908. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Han, L.; Zhang, L.; Zhu, J.; Liu, X. Polyesters derived from itaconic acid for the properties and bio-based content enhancement of soybean oil-based thermosets. Green Chem. 2015, 17, 2383–2392. [Google Scholar] [CrossRef]
- Chen, T.; Tian, S.; Xie, Z.; Guo, Z.-X.; Xu, J.; Guo, B.-H. Two new approaches based on dynamic carboxyl–hydroxyl or hydroxyl–carboxyl transformation for high molecular weight poly(butylene maleate). Polym. Chem. 2020, 11, 5884–5892. [Google Scholar] [CrossRef]
- Verissimo Lobo, V.T.; Pacheco Ortiz, R.W.; Gonçalves, V.O.O.; Cajaiba, J.; Kartnaller, V. Kinetic Modeling of Maleic Acid Isomerization to Fumaric Acid Catalyzed by Thiourea Determined by Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy. Org. Process. Res. Dev. 2020, 24, 988–996. [Google Scholar] [CrossRef]
- Li, Q.; Tao, W.; Li, A.; Zhou, Q.; Shuang, C. Poly (4-vinylpyridine) catalyzed isomerization of maleic acid to fumaric acid. Appl. Catal. A-Gen. 2014, 484, 148–153. [Google Scholar] [CrossRef]
- Lam, Y.P.; Lam, Z.; Yeung, Y.Y. Zwitterion-Catalyzed Isomerization of Maleic to Fumaric Acid Diesters. J. Org. Chem. 2021, 86, 1183–1190. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Li, C.; Xiao, Y.; Zhang, D.; Guan, G.; Zhu, W. Synthesis, characterization and properties of novel linear poly(butylene fumarate) bearing reactive double bonds. Polymer 2013, 54, 631–638. [Google Scholar] [CrossRef]
- Dunjic, B.; Sepulchre, M.O.; Sepulchre, M.; Spassky, N.; Djonlagic, J. Synthesis and rheological study of some maleic acid and fumaric acid stereoregular polyesters, 10-Synthesis and characterization of alpha, omega-dihydroxyoligo(alkylene maleate)s. Macromol. Chem. Phys. 1998, 199, 1051–1055. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, Y.C.; Lai, T.H.; Chiu, S.H.; Uchibe, N.; Lin, H.W.; Chiu, W.Y.; Tung, S.H.; Jeng, R.J. Facile synthesis toward self-dispersible waterborne comb-like Poly(hydroxyaminoethers). Polymer 2020, 196, 122464. [Google Scholar] [CrossRef]
- Martinet, F.; Guillot, J. Copolymerization of α-methylstyrene with methyl methacrylate. III. Emulsion process: Experimental data on kinetics, particle size, composition, molecular weight, and glass transition temperature. J. Appl. Polym. Sci. 1999, 72, 1627–1643. [Google Scholar] [CrossRef]
- Querol, N.; Barreneche, C.; Cabeza, L. Storage Stability of Bimodal Emulsions vs. Monomodal Emulsions. Appl. Sci. 2017, 7, 1267. [Google Scholar] [CrossRef] [Green Version]
- De Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Wajnryb, E.; Dahler, J.S. The Viscosity of Polymerically Stabilized Dispersions of Spherical Colloid Particles. J. Colloid Interface Sci. 1999, 217, 259–268. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Branched polyacrylamides: Synthesis and effect of molecular architecture on solution rheology. Eur. Polym. J. 2013, 49, 3289–3301. [Google Scholar] [CrossRef]
- Lei, L.; Xia, Z.; Ou, C.; Zhang, L.; Zhong, L. Effects of crosslinking on adhesion behavior of waterborne polyurethane ink binder. Prog. Org. Coat. 2015, 88, 155–163. [Google Scholar] [CrossRef]
- Koo, J.M.; Hwang, S.Y.; Yoon, W.J.; Lee, Y.G.; Kim, S.H.; Im, S.S. Structural and thermal properties of poly(1,4-cyclohexane dimethylene terephthalate) containing isosorbide. Polym. Chem. 2015, 6, 6973–6986. [Google Scholar] [CrossRef]
- Cai, X.; Yang, X.; Zhang, H.; Wang, G. Modification of biodegradable poly(butylene carbonate) with 1,4-cyclohexanedimethylene to enhance the thermal and mechanical properties. Polym. Degrad. Stab. 2017, 143, 35–41. [Google Scholar] [CrossRef]
- Hahm, S.; Kim, J.-S.; Yun, H.; Park, J.H.; Letteri, R.A.; Kim, B.J. Bench-Scale Synthesis and Characterization of Biodegradable Aliphatic–Aromatic Random Copolymers with 1,4-Cyclohexanedimethanol Units Toward Sustainable Packaging Applications. ACS Sustain. Chem. Eng. 2019, 7, 4734–4743. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Giliopoulos, D.J.; Stergiou, C.A. Correlation between chemical and solid-state structures and enzymatic hydrolysis in novel biodegradable polyesters. The case of poly(propylene alkanedicarboxylate)s. Macromol. Biosci. 2008, 8, 728–740. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wu, G.F.; Zhang, H.X. Effects of the reagent molar ratio on the phase separation and properties of waterborne polyurethane for application in a water-based ink binder. J. Appl. Polym. Sci. 2017, 134, 45406. [Google Scholar] [CrossRef]
- Wang, L.; Yan, F.A. Research on Synthesis of Hydroxy Waterborne Polyester-acrylic Resin Hybrid. China Coatings 2019, 34, 41–45. [Google Scholar] [CrossRef]
- Rowe, M.D.; Eyiler, E.; Walters, K.B. Hydrolytic degradation of bio-based polyesters: Effect of pH and time. Polym. Test. 2016, 52, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Bayramoğlu, G.; Kahraman, M.V.; Kayaman-Apohan, N.; Güngör, A. The coating performance of adipic acid modified and methacrylated bisphenol- A based epoxy oligomers. Polym. Adv. Technol. 2007, 18, 173–179. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Zhu, J.; Liu, X. High bio-based content waterborne UV-curable coatings with excellent adhesion and flexibility. Prog. Org. Coat. 2015, 87, 197–203. [Google Scholar] [CrossRef]
- Dong, X.; Ren, J.; Duan, Y.; Wu, D.; Lin, L.; Shi, J.; Jia, R.; Xu, X.; He, X. Preparation and properties of green UV-curable itaconic acid cross-linked modified waterborne polyurethane coating. J. Appl. Polym. Sci. 2021, 139, e52042. [Google Scholar] [CrossRef]
- Gogoi, G.; Gogoi, S.; Karak, N. Dimer acid based waterborne hyperbranched poly(ester amide) thermoset as a sustainable coating material. Prog. Org. Coat. 2017, 112, 57–65. [Google Scholar] [CrossRef]
- Gao, C.; Han, S.; Zhang, D.; Wang, B.; Wang, C.; Wu, Y.; Liu, Y. A facile preparation of UV-cured films from waterborne unsaturated polyester via click reaction. Prog. Org. Coat. 2018, 124, 232–239. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Castelvetro, V.; Chiantore, O. Surface Monitoring of Surfactant Phase Separation and Stability in Waterborne Acrylic Coatings. Chem. Mater. 2007, 19, 6107–6113. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, H.; Yang, L.; Sheng, J.; Fu, H. Robust Hyperbranched Polyester-Based Anti-Smudge Coatings for Self-Cleaning, Anti-Graffiti, and Chemical Shielding. ACS Appl. Mater. Inter. 2019, 11, 14305–14312. [Google Scholar] [CrossRef] [PubMed]











| Sample (a) | Catalyst | Reaction Temperature (°C) | Diacid Dosage (mol) | Diol Dosage (mol) | Functionality (b) | |||
|---|---|---|---|---|---|---|---|---|
| MA | HA | CHDM | HG | NPG | ||||
| Entry-1L | DBTDL | 180 | 0.05 | 0.05 | 0 | 0.084 | 0.021 | / |
| Entry-2L | Ti(OBu)4 | 180 | 0.05 | 0.05 | 0 | 0.084 | 0.021 | / |
| Entry-3L | TsOH | 180 | 0.05 | 0.05 | 0 | 0.084 | 0.021 | / |
| Entry-3C | 4.94 | |||||||
| Entry-4L | TsOH | 140 | 0.05 | 0.05 | 0 | 0.084 | 0.021 | / |
| Entry-5L | TsOH | 170 | 0.05 | 0.05 | 0 | 0.084 | 0.021 | / |
| Entry-6L | TsOH | 190 | 0.05 | 0.05 | 0 | 0.084 | 0.021 | / |
| Entry-7L | TsOH | 180 | 0.03 | 0.07 | 0 | 0.084 | 0.021 | / |
| Entry-7C | 3.52 | |||||||
| Entry-8L | TsOH | 180 | 0.04 | 0.06 | 0 | 0.084 | 0.021 | / |
| Entry-8C | 4.16 | |||||||
| Entry-9L | TsOH | 180 | 0.06 | 0.04 | 0 | 0.084 | 0.021 | / |
| Entry-9C | 5.63 | |||||||
| Entry-10L | TsOH | 180 | 0.07 | 0.03 | 0 | 0.084 | 0.021 | / |
| Entry-10C | 7.48 | |||||||
| Entry-11L | TsOH | 180 | 0.05 | 0.05 | 0.021 | 0.063 | 0.021 | / |
| Entry-11C | 4.48 | |||||||
| Entry-12L | TsOH | 180 | 0.05 | 0.05 | 0.042 | 0.042 | 0.021 | / |
| Entry-12C | 4.82 | |||||||
| Entry-13L | TsOH | 180 | 0.05 | 0.05 | 0.063 | 0.021 | 0.021 | / |
| Entry-13C | 5.15 | |||||||
| Entry-14L | TsOH | 180 | 0.05 | 0.05 | 0.084 | 0 | 0.021 | / |
| Entry-14C | 5.21 | |||||||
| Sample | Catalyst | cis:trans | Mn (g mol−1) | Mw/Mn | Appearance |
|---|---|---|---|---|---|
| Entry-1L | DBTDL | 83:17 | 2820 | 2.12 | Light yellow transparent |
| Entry-2L | Ti(OBu)4 | 62:38 | 14330 | 2.25 | Dark brown transparent |
| Entry-3L | TsOH | 75:25 | 5870 | 2.05 | Yellow transparent |
| Sample | MA Content | Dispersion Appearance | Pencil Hardness | Adhesion | Water Resistance | Storage Stability |
|---|---|---|---|---|---|---|
| Entry-7C | 30% | Milky white, untransparent | B | 1° | Transparent | >180 d |
| Entry-8C | 40% | Light yellow, transparent | B | 1° | Transparent | >180 d |
| Entry-3C | 50% | Light yellow, transparent | B | 1° | Transparent | >180 d |
| Entry-9C | 60% | Flocculation | / | / | / | <30 d |
| Entry-10C | 70% | Flocculation | / | / | / | <30 d |
| Sample | CHDM Content | Dispersion Appearance | Pencil Hardness | Adhesion | Water Resistance | Storage Stability |
|---|---|---|---|---|---|---|
| Entry-3C | 0% | Light yellow, transparent | B | 1° | Transparent | >180 d |
| Entry-11C | 20% | Light yellow, transparent | HB | 1° | Transparent | >180 d |
| Entry-12C | 40% | Light yellow, transparent | H | 0° | Transparent | >180 d |
| Entry-13C | 60% | Light yellow, transparent | H | 0° | Transparent | >180 d |
| Entry-14C | 80% | Dark yellow, transparent | H | 0° | Transparent | >180 d |
| CHDM Content | 0% | 20% | 40% | 60% | 80% |
|---|---|---|---|---|---|
| σ (MPa) | 3.1 ± 0.2 | 5.2 ± 0.2 | 5.9 ± 0.2 | 5.8 ± 0.3 | 5.2 ± 0.3 |
| Ε (MPa) | 4.0 ± 0.4 | 10.1 ± 0.3 | 5.8 ± 0.5 | 6.7 ± 0.8 | 4.8 ± 0.7 |
| ε (%) | 70.3 ± 0.8 | 51.6 ± 0.6 | 88.1 ± 0.4 | 73.2 ± 0.9 | 90.5 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Gong, L.; Gong, S. A New Approach Utilizing Aza-Michael Addition for Hydrolysis-Resistance Non-Ionic Waterborne Polyester. Polymers 2022, 14, 2655. https://doi.org/10.3390/polym14132655
Fu H, Gong L, Gong S. A New Approach Utilizing Aza-Michael Addition for Hydrolysis-Resistance Non-Ionic Waterborne Polyester. Polymers. 2022; 14(13):2655. https://doi.org/10.3390/polym14132655
Chicago/Turabian StyleFu, Hao, Linbo Gong, and Shuling Gong. 2022. "A New Approach Utilizing Aza-Michael Addition for Hydrolysis-Resistance Non-Ionic Waterborne Polyester" Polymers 14, no. 13: 2655. https://doi.org/10.3390/polym14132655
APA StyleFu, H., Gong, L., & Gong, S. (2022). A New Approach Utilizing Aza-Michael Addition for Hydrolysis-Resistance Non-Ionic Waterborne Polyester. Polymers, 14(13), 2655. https://doi.org/10.3390/polym14132655