Superhydrophobic Nanosilica Decorated Electrospun Polyethylene Terephthalate Nanofibers for Headspace Solid Phase Microextraction of 16 Organochlorine Pesticides in Environmental Water Samples
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Gas Chromatography
2.3. Instrumentation
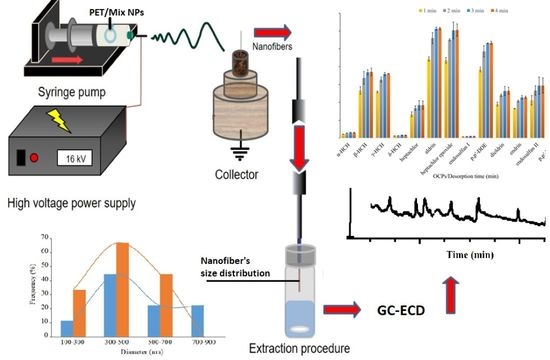
2.4. Electrospinning of Nanofibers
2.5. The Procedure
3. Results
3.1. Effect of Adsorbent Composition
3.2. Effect of Electrospinning Flow Rate
3.3. Characterization
3.4. Effect of Parameters on Extraction Efficiency
3.4.1. Effect of Salt Concentration
3.4.2. Effect of Extraction Temperature
3.4.3. Effect of Extraction Time
3.4.4. Effect of Desorption Temperature and Time
3.5. Method Validation
3.6. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moreno, D.V.; Ferrera, Z.S.; Santana Rodríguez, J.J. Microwave assisted micellar extraction coupled with solid phase microextraction for the determination of organochlorine pesticides in soil samples. Anal. Chim. Acta. 2006, 571, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, X.; Yuan, J.; Jiang, T.; Fu, S.; Xu, X. A novel headspace solid-phase microextraction method for the exact determination of organochlorine pesticides in environmental soil samples. Anal. Bioanal. Chem. 2006, 384, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef]
- Lallas, P.L. The Stockholm Convention on persistent organic pollutants. Am. J. Int. Law 2001, 95, 692–708. [Google Scholar] [CrossRef]
- WHO (Water Health Organization). The Use of DDT in Malaria Vector Control. WHO Position Statement. Available online: http://whqlibdoc.who.int/hq/2011/WHO_HTM_GMP_2011_eng.pdf (accessed on 17 July 2017).
- Choi, M.; Lee, I.-S.; Jung, R.-H. Rapid determination of organochlorine pesticides in fish using selective pressurized liquid extraction and gas chromatography–mass spectrometry. Food Chem. 2016, 205, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duodu, G.O.; Goonetilleke, A.; Ayoko, G.A. Optimization of in-cell accelerated solvent extraction technique for the determination of organochlorine pesticides in river sediments. Talanta 2016, 150, 278–285. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C.; Daudc, M.; Alsharaa, A. Evaluation of layered double hydroxide/graphene hybrid as a sorbent in membrane-protected stir-bar supported micro-solid-phase extraction for determination of organochlorine pesticides in urine samples. J. Chromatogr. A 2017, 1489, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Z.; Wu, R.; Wang, Z.; Chen, X.; Chan, T.-W.D. Magnetic porous carbon derived from a bimetallic metal–organic framework for magnetic solid-phase extraction of organochlorine pesticides from drinking and environmental water samples. J. Chromatogr. A 2017, 1479, 55–61. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Sereshti, H. One-step green synthesis of β-cyclodextrin/iron oxide-reduced graphene oxide nanocomposite with high supramolecular recognition capability: Application for vortex-assisted magnetic solid phase extraction of organochlorine pesticides residue from honey samples. J. Chromatogr. A 2017, 1485, 32–43. [Google Scholar]
- Xie, L.; Liu, S.; Han, Z.; Jiang, R.; Zhu, F.; Xu, W.; Su, C.; Ouyang, G. Amine-functionalized MIL-53 (Al)-coated stainless steel fiber for efficient solid-phase microextraction of synthetic musks and organochlorine pesticides in water samples. Anal. Bioanal. Chem. 2017, 409, 5239–5247. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Spietelun, A.; Kloskowski, A.; Chrzanowski, W.; Namieśnik, J. Understanding solid-phase microextraction: Key factors influencing the extraction process and trends in improving the technique. Chem. Rev. 2012, 113, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namieśnik, J. Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524–4537. [Google Scholar] [CrossRef] [PubMed]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. TrAC Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. TrAC Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Aziz-zanjani, M.O.; Mehdinia, A. A review on procedures for the preparation of coatings for solid phase microextraction. Microchim. Acta 2014, 181, 1169–1190. [Google Scholar] [CrossRef]
- Mehdinia, A.; Aziz-zanjani, M.O.; Mwcnts, P.E.G. Recent advances in nanomaterials utilized in fiber coatings for solid-phase microextraction. TrAC Trends Anal. Chem. 2013, 42, 205–215. [Google Scholar] [CrossRef]
- Mehdinia, A.; Mousavi, M.F. Enhancing extraction rate in solid-phase microextraction by using nanostructured polyaniline coating. J. Sep. Sci. 2008, 31, 3565–3572. [Google Scholar] [CrossRef]
- Wang, J.-X.; Jiang, D.-Q.; Gu, Z.-Y.; Yan, X.-P. Multiwalled carbon nanotubes coated fibers for solid-phase microextraction of polybrominated diphenyl ethers in water and milk samples before gas chromatography with electron-capture detection. J. Chromatogr. A 2006, 1137, 8–14. [Google Scholar] [CrossRef]
- Tian, J.; Xu, J.; Zhu, F.; Lu, T.; Su, C.; Ouyang, G. Application of nanomaterials in sample preparation. J. Chromatogr. A 2013, 1300, 2–16. [Google Scholar] [CrossRef]
- Liu, Y.; Su, G.; Zhang, B.; Jiang, G.; Yan, B. Nanoparticle-based strategies for detection and remediation of environmental pollutants. Anal. 2011, 136, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Yuan, D. Preparation of solid-phase microextraction fiber coated with single-walled carbon nanotubes by electrophoretic deposition and its application in extracting phenols from aqueous samples. J. Chromatogr. A 2009, 1216, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Aghakhani, A. Novel nanofiber coatings prepared by electrospinning technique for headspace solid-phase microextraction of chlorobenzenes from environmental samples. Anal. Methods 2011, 6, 1284–1289. [Google Scholar] [CrossRef]
- Zewe, J.W.; Steach, J.K.; Olesik, S.V. Electrospun fibers for solid-phase microextraction, Anal. Chem. 82 (2010) 5341–5348.
- Sereshti, H.; Amini, F.; Najarzadekan, H. Electrospun polyethylene terephthalate (PET) nanofibers as a new adsorbent for micro-solid phase extraction of chromium (VI) in environmental water samples. RSC Adv. 2015, 5, 89195–89203. [Google Scholar] [CrossRef]
- Eskandarpour, N.; Sereshti, H.; Najarzadekan, H.; Gaikani, H. Polyurethane/polystyrene-silica electrospun nanofibrous composite for the headspace solid-phase microextraction of chlorophenols coupled with gas chromatography. J. Sep. Sci. 2016, 39, 4637–4644. [Google Scholar] [CrossRef]
- Sereshti, H.; Bakhtiari, S.; Najarzadekan, H.; Samadi, S. Electrospun polyethylene terephthalate/graphene oxide nanofibrous membrane followed by HPLC for the separation and determination of tamoxifen in human blood plasma. J. Sep. Sci. 2017, 40, 3383–3391. [Google Scholar] [CrossRef]
- Eskandarpour, N.; Sereshti, H. Electrospun polycaprolactam-manganese oxide fiber for headspace solid phase microextraction of phthalate esters in water samples. Chemosphere 2018, 191, 36–430. [Google Scholar] [CrossRef]
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S. Electrospun nanofibers as sorptive phases in microextraction. TrAC Trends Anal. Chem. 2016, 84, 3–11. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Akoz, E.; Memon, S.; Yilmaz, M. Synthesis of amino-substituted p-tert-butylcalix [4] arene for the removal of chicago sky blue and tropaeolin 000 azo dyes from aqueous environment. Water Air Soil Pollut. 2013, 224, 1424. [Google Scholar] [CrossRef]
- Yavuz, H.; Guler, G.O.; Aktumsek, A.; Cakmak, Y.S.; Ozparlak, H. Determination of some organochlorine pesticide residues in honeys from Konya, Turkey. Environ. Monit. Assess. 2010, 168, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Penalver, R.; Aguinaga, N.; Hernández-Córdoba, M. Solid-phase microextraction and gas chromatography with atomic emission detection for multiresidue determination of pesticides in honey. Anal. Chim. Acta 2006, 562, 9–15. [Google Scholar] [CrossRef]
- Mokbel, H.; Al Dine, E.J.; Elmoll, A.; Liaud, C.; Millet, M. Simultaneous analysis of organochlorine pesticides and polychlorinated biphenyls in air samples by using accelerated solvent extraction (ASE) and solid-phase micro-extraction (SPME) coupled to gas chromatography dual electron capture detection. Environ. Sci. Pollut. Res. 2016, 23, 8053–8063. [Google Scholar] [CrossRef] [PubMed]








| Compound | LOD a | LOQ b | LDR c | R2 | RSD d | RSD e | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intraday | Interday | Fiber to Fiber | Intraday | Interday | Fiber to Fiber | |||||
| α-HCH | 30 | 80 | 80–10,000 | 0.9967 | 6.3 | 8.6 | 7.5 | 2.2 | 6.4 | 5.4 |
| β-HCH | 20 | 50 | 50–5000 | 0.9914 | 5.3 | 1.7 | 3.5 | 3.1 | 2.4 | 2.4 |
| γ-HCH | 20 | 50 | 50–5000 | 0.9914 | 4.8 | 9.5 | 7.1 | 3.2 | 7.0 | 6.3 |
| δ-HCH | 80 | 200 | 200–10,000 | 0.9983 | 8.1 | 10.6 | 4.8 | 8.2 | 8.8 | 11.9 |
| Heptachlor | 20 | 50 | 50–5000 | 0.9907 | 10.5 | 7.3 | 8.9 | 5.5 | 6.0 | 6.4 |
| Aldrin | 3 | 8 | 8–2000 | 0.9977 | 6.2 | 4.5 | 5.4 | 4.0 | 3.9 | 4.2 |
| Heptachlor epoxide | 3 | 8 | 8–2000 | 0.9930 | 5.5 | 2.6 | 4.0 | 5.5 | 2.7 | 4.0 |
| Endosulfan I | 80 | 200 | 200–10,000 | 0.9987 | 10.9 | 10.3 | 11.6 | 5.7 | 10.6 | 12.0 |
| p,p′-DDE | 3 | 8 | 8–2000 | 0.9989 | 7.2 | 6.5 | 6.8 | 2.6 | 4.2 | 4.5 |
| Dieldrin | 30 | 80 | 80–5000 | 0.9909 | 11.3 | 7.6 | 11.5 | 4.2 | 6.0 | 5.9 |
| Endrin | 30 | 80 | 80–5000 | 0.9987 | 9.8 | 3.1 | 6.5 | 1.7 | 3.4 | 2.4 |
| Endosulfan II | 30 | 80 | 80–5000 | 0.9932 | 12.3 | 8.1 | 10.2 | 5.6 | 4.3 | 6.9 |
| p,p′-DDD | 30 | 80 | 80–2000 | 0.9912 | 12.4 | 8.5 | 5.4 | 10.0 | 8.8 | 9.3 |
| Endrin aldehyde | 30 | 80 | 80–2000 | 0.9942 | 13.8 | 8.0 | 5.4 | 9.5 | 11.0 | 12.3 |
| Endosulfan sulfate | 30 | 80 | 80–5000 | 0.9921 | 11.4 | 9.3 | 7.3 | 8.5 | 6.6 | 11.4 |
| p,p′-DDT | 30 | 80 | 80–5000 | 0.9965 | 11.3 | 10.5 | 11.4 | 11.3 | 11.8 | 13.4 |
| Compound | Industrial Water a (RR%) b | Sewage Water c (RR%) | Tap Water (RR%) | River Water d (RR%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 e | RSD% | S2 f | RSD% | S1 | RSD% | S2 | RSD% | S1 | RSD% | S2 | RSD% | S1 | RSD% | S2 | RSD% | |
| α-HCH | 100.3 | 6 | 99.6 | 6.6 | 93.7 | 7.8 | 99.6 | 6.6 | 90.8 | 6.3 | 99.4 | 2.2 | 96.5 | 11.3 | 101 | 4.9 |
| β-HCH | 114.9 | 2.9 | 97.7 | 2.4 | 98 | 1.5 | 97.7 | 2.4 | 89.8 | 5.3 | 99.8 | 3.1 | 105.1 | 2.5 | 99.3 | 3.9 |
| γ-HCH | 88.8 | 3.5 | 93.3 | 7.7 | 103.3 | 1.5 | 93.3 | 7.7 | 91.2 | 4.8 | 100.1 | 3.2 | 90.3 | 1.7 | 96.4 | 5.0 |
| δ-HCH | 95.4 | 18 | 95.1 | 13.6 | 120.7 | 7.3 | 95.1 | 13.6 | 103.1 | 48.1 | 100.4 | 8.2 | 113.2 | 17.0 | 98.1 | 9.8 |
| Heptachlor | 98.9 | 11 | 97.1 | 6.9 | 98.4 | 10.1 | 97.1 | 6.9 | 100.8 | 10.5 | 99.6 | 5.5 | 103.1 | 6.9 | 100.9 | 6.3 |
| Aldrin | 96.0 | 1.3 | 98 | 4.6 | 98.2 | 5.2 | 98 | 4.6 | 102.3 | 6.2 | 100 | 4.0 | 101.5 | 4.9 | 100.5 | 4.3 |
| Heptachlorepoxide | 98.9 | 5.9 | 96.7 | 1.7 | 101.3 | 4.4 | 96.7 | 1.7 | 100.1 | 5.5 | 99.7 | 5.5 | 102.0 | 3.1 | 96.0 | 1.7 |
| Endosulfan I | 100 | 10.4 | 96.2 | 13.1 | 116.1 | 10.7 | 96.2 | 13.1 | 95.3 | 10.9 | 100.5 | 5.7 | 102.5 | 9.2 | 101.1 | 5.2 |
| p,p′-DDE | 95.2 | 1.3 | 99.6 | 4.2 | 98.9 | 7.0 | 99.6 | 4.2 | 96.0 | 7.2 | 100.3 | 2.6 | 101.5 | 6.1 | 102.2 | 2.2 |
| Dieldrin | 87.3 | 7.0 | 97.1 | 6.4 | 90.9 | 4.8 | 97.1 | 6.4 | 92.8 | 21.3 | 99.4 | 4.2 | 105.8 | 14.7 | 96.5 | 5.5 |
| Endrin | 103 | 7.3 | 100.8 | 3.7 | 99.9 | 4.1 | 100.8 | 3.7 | 101.4 | 9.8 | 100.5 | 1.7 | 100.3 | 9.8 | 99.6 | 3.0 |
| Endosulfan II | 97.9 | 13.7 | 96.0 | 5.1 | 95.7 | 10.1 | 96 | 5.1 | 99.7 | 12.3 | 100.2 | 5.6 | 103.2 | 8.7 | 95.8 | 5.2 |
| p,p′-DDD | 88.3 | 7.0 | 96.1 | 11.0 | 92.6 | 13.8 | 96.1 | 11 | 90.0 | 22.4 | 99.6 | 10.0 | 106.1 | 15.5 | 96.4 | 11.5 |
| Endrin aldehyde | 81.3 | 10.1 | 100 | 13.2 | 94.1 | 12 | 100 | 13.2 | 97.8 | 23.8 | 101.3 | 9.5 | 104.7 | 16.6 | 99.4 | 12.2 |
| Endosulfan sulfate | 82.4 | 17 | 98.3 | 8.0 | 79.1 | 14.5 | 98.3 | 8.0 | 98.5 | 14.4 | 100.8 | 8.5 | 102.3 | 11.2 | 99.2 | 9.6 |
| p,p′-DDT | 78.7 | 7.0 | 100.6 | 18 | 88.4 | 14.1 | 100.6 | 18 | 93.1 | 21.3 | 100.1 | 11.3 | 99.9 | 16.2 | 97.3 | 12.8 |
| Methods | Sorbent | LOD a | LDR b | Extraction Time (min) | RR% c | Ref |
|---|---|---|---|---|---|---|
| SPME | PET/NanoSiO2 | 3–80 | 8–10,000 | 10 | 78–120 | Current study |
| SPE-GC/ECD | Florisil | 400–2000 | 5–1000 | - | 77–105 | [33] |
| SMPE | PDMS d/PA e | 20–80 | 50–1000 | 20 | 91.4 (average) | [34] |
| SB-μ-SPE | Hydroxide/graphene | 300–1400 | 1000–200,000 | 20 | 84.2–100.2 | [8] |
| ASE f & SPME | PDMS/PA | 0.2–4.9 (ng m−3) | 50–3000 (ng m−3) | 40 | - | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najarzadekan, H.; Sereshti, H.; Ahmad, I.; Shahabuddin, S.; Rashidi Nodeh, H.; Sridewi, N. Superhydrophobic Nanosilica Decorated Electrospun Polyethylene Terephthalate Nanofibers for Headspace Solid Phase Microextraction of 16 Organochlorine Pesticides in Environmental Water Samples. Polymers 2022, 14, 3682. https://doi.org/10.3390/polym14173682
Najarzadekan H, Sereshti H, Ahmad I, Shahabuddin S, Rashidi Nodeh H, Sridewi N. Superhydrophobic Nanosilica Decorated Electrospun Polyethylene Terephthalate Nanofibers for Headspace Solid Phase Microextraction of 16 Organochlorine Pesticides in Environmental Water Samples. Polymers. 2022; 14(17):3682. https://doi.org/10.3390/polym14173682
Chicago/Turabian StyleNajarzadekan, Hamid, Hassan Sereshti, Irfan Ahmad, Syed Shahabuddin, Hamid Rashidi Nodeh, and Nanthini Sridewi. 2022. "Superhydrophobic Nanosilica Decorated Electrospun Polyethylene Terephthalate Nanofibers for Headspace Solid Phase Microextraction of 16 Organochlorine Pesticides in Environmental Water Samples" Polymers 14, no. 17: 3682. https://doi.org/10.3390/polym14173682
APA StyleNajarzadekan, H., Sereshti, H., Ahmad, I., Shahabuddin, S., Rashidi Nodeh, H., & Sridewi, N. (2022). Superhydrophobic Nanosilica Decorated Electrospun Polyethylene Terephthalate Nanofibers for Headspace Solid Phase Microextraction of 16 Organochlorine Pesticides in Environmental Water Samples. Polymers, 14(17), 3682. https://doi.org/10.3390/polym14173682