Computational Foretelling and Experimental Implementation of the Performance of Polyacrylic Acid and Polyacrylamide Polymers as Eco-Friendly Corrosion Inhibitors for Copper in Nitric Acid
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Techniques
2.3. Theoretical Studies
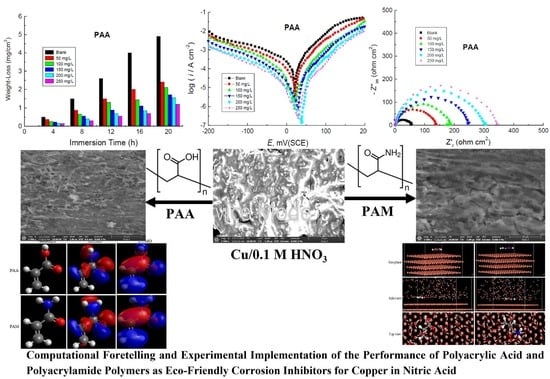
3. Results and Discussion
3.1. WL Measurements
3.1.1. Effect of Polymers’ Concentrations
3.1.2. Effect of Corrosive Medium Concentration
3.1.3. Effect of Temperature
3.1.4. Adsorption Consideration
3.1.5. Thermodynamic Parameters
3.1.6. Kinetic Parameters
3.1.7. Kinetics of Corrosion and Its Inhibition
3.2. PDP Measurements
3.3. EIS Measurements
3.4. Surface Examination
3.5. Theoretical Studies
3.5.1. Quantum Chemical Calculations
3.5.2. Molecular Dynamic Simulations
3.6. Proposed Corrosion Inhibition Mechanisms of Copper in Nitric Acid Solution
4. Conclusions
- Cu corrosion in 1.0 M HNO3 medium and its inhibition using poly(acrylic acid) and polyacrylamide polymers were investigated using various tools.
- The tested polymers were set to be efficient inhibitors for Cu corrosion in 1.0 M HNO3 medium, and the values of inhibition efficiencies of poly(acrylic acid) are slightly higher than those recorded for polyacrylamide.
- Thermodynamic and kinetic parameters were determined that sustain the mechanism of physical adsorption of the tested polymers.
- The proposed adsorption of the polymeric molecules on the copper surface obeyed the Langmuir isotherm.
- The tested polymers were found to perform as mixed-type inhibitors with anodic priority.
- The kinetics and mechanisms of copper corrosion and its inhibition were investigated.
- There is a good agreement between all employed techniques.
- The creation of connections between inhibitors’ active sites and copper atoms was predicted by DFT simulations, and their increased affinity for metal surface was supported by their tight and parallel distribution over its surface. The chemicals used in this investigation demonstrated to be potential corrosion inhibitors.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Lateef, H.M.A.; El-Beltagi, H.S.; Mohamed, M.E.M.; Kandeel, M.; Bakir, E.; Toghan, A.; Shalabi, K.; Tantawy, A.H.; Khalaf, M.M. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Fawzy, A.; Toghan, A. Inhibition Evaluation of Chromotrope Dyes for the Corrosion of Mild Steel in an Acidic Environment: Thermodynamic and Kinetic Aspects. ACS Omega 2021, 6, 4051–4061. [Google Scholar] [CrossRef]
- Fawzy, A.; Zaafarany, I.A.; Ali, H.M.; Abdallah, M. New synthesized amino acids-based surfactants as efficient inhibitors for corrosion of mild steel in hydrochloric acid medium: Kinetics and thermodynamic approach. Int. J. Electrochem. Sci. 2018, 13, 4575–4600. [Google Scholar] [CrossRef]
- Heikal, M.; Ali, A.; Ibrahim, B.; Toghan, A. Electrochemical and physico-mechanical characterizations of fly ash-composite cement. Constr. Build. Mater. 2020, 243, 118309. [Google Scholar] [CrossRef]
- Toghan, A.; Fawzy, A.; Alqarni, N.; Abdelkader, A.; Alakhras, A.I. Inhibition Effects of Citrulline and Glutamine for Mild Steel Corrosion in Sulfuric Acid Environment: Thermodynamic and Kinetic Aspects. Int. J. Electrochem. Sci. 2021, 16, 211118. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Hawsawi, H. Estimation of water-soluble polymeric materials (Poloxamer and Pectin) as corrosion inhibitors for carbon steel in acidic medium. Int. J. Electrochem. Sci. 2020, 15, 8129–8144. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Hawsawi, H. Maltodextrin and chitosan polymers as inhibitors for the corrosion of carbon steel in 1.0 M hydrochloric acid. Int. J. Electrochem. Sci. 2020, 15, 5650–5663. [Google Scholar] [CrossRef]
- Abdallah, M.; Al-Gorair, A.S.; Fawzy, A.; Hawsawi, H.; Hameed, R.S.A. Enhancement of adsorption and anticorrosion performance of two polymeric compounds for the corrosion of SABIC carbon steel in hydrochloric acid. J. Adhes. Sci. Technol. 2021, 36, 35–53. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Alfakeer, M.; Altass, H.M.; Althagafi, I.I.; el Ossaily, Y.A. Performance of unprecedented synthesized biosurfactants as green inhibitors for the corrosion of mild steel-37-2 in neutral solutions: A mechanistic approach. Green Chem. Lett. Rev. 2021, 14, 488–499. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Alfakeer, M.; Ali, H.M. Corrosion inhibition of Sabic iron in different media using synthesized sodium N-dodecyl arginine surfactant. Int. J. Electrochem. Sci. 2019, 14, 2063–2084. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Alfakeer, M.; Altass, H.M. Expired azithromycin and roxithromycin drugs as environmentally friendly inhibitors for mild steel corrosion in H2SO4 solutions. Green Chem. Lett. Rev. 2021, 14, 509–518. [Google Scholar] [CrossRef]
- Alfakeer, M.; Abdallah, M.; Fawzy, A. Corrosion inhibition effect of expired ampicillin and flucloxacillin drugs for mild steel in aqueous acidic medium. Int. J. Electrochem. Sci. 2020, 15, 3283–3297. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Alfakeer, M. Inhibition potentials and adsorption performance of two sulfonylurea antibiotic expired drugs on the corrosion of mild steel in 0.5 M H2SO4. Int. J. Electrochem. Sci. 2020, 15, 10289–10303. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Al Bahir, A. The effect of expired acyclovir and omeprazole drugs on the inhibition of Sabic iron corrosion in HCl solution. Int. J. Electrochem. Sci. 2020, 15, 4739–4753. [Google Scholar] [CrossRef]
- Chibowski, S.; Krupa, M. Study of the Influence of Poly(acrylic acid) and Polyacrylamide on the Electrochemical Properties of the ZrO/Solution Interface. Adsorpt. Sci. Technol. 1999, 17, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Farag, A.A.; Toghan, A.; Mostafa, M.S.; Lan, C.; Ge, G. Environmental Remediation through Catalytic Inhibition of Steel Corrosion by Schiff’s Bases: Electrochemical and Biological Aspects. Carbohydr. Polym. 2022, 12, 838. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Takahashi, A. Polymer adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1992, 37, 219–317. [Google Scholar] [CrossRef]
- Toghan, A.; Gouda, M.; Shalabi, K.; El-Lateef, H. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers 2021, 13, 2275. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126894. [Google Scholar] [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef]
- Jeyaprabha, C.; Sathiyanarayanan, S.; Phani, K.; Venkatachari, G. Influence of poly(aminoquinone) on corrosion inhibition of iron in acid media. Appl. Surf. Sci. 2005, 252, 966–975. [Google Scholar] [CrossRef]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Umoren, S.; Li, Y.; Wang, F. Influence of iron microstructure on the performance of polyacrylic acid as corrosion inhibitor in sulfuric acid solution. Corros. Sci. 2011, 53, 1778–1785. [Google Scholar] [CrossRef]
- Cui, M.; Yu, Y.; Zheng, Y. Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots. Polymers 2021, 13, 1923. [Google Scholar] [CrossRef]
- Umoren, S.; Li, Y.; Wang, F. Electrochemical study of corrosion inhibition and adsorption behaviour for pure iron by polyacrylamide in H2SO4: Synergistic effect of iodide ions. Corros. Sci. 2010, 52, 1777–1786. [Google Scholar] [CrossRef]
- Petrunin, M.; Rybkina, A.; Yurasova, T.; Maksaeva, L. Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals. Polymers 2022, 14, 4428. [Google Scholar] [CrossRef]
- Srivastava, V.; Banerjee, S.; Singh, M.M. Inhibitive effect of polyacrylamide grafted with fenugreek mucilage on corrosion of mild steel in 0.5 M H2SO4 at 35 °C. J. Appl. Polym. Sci. 2009, 116, 810–816. [Google Scholar] [CrossRef]
- Chamovska, D.; Cvetkovska, M.; Grchev, T. Corrosion inhibition of iron in hydrochloric acid by polyacrylamide. J. Serbian Chem. Soc. 2007, 72, 687–698. [Google Scholar] [CrossRef]
- Farag, A.; Mohamed, E.; Toghan, A. The new trends in corrosion control using superhydrophobic surfaces: A review. Corros. Rev. 2022; in press. [Google Scholar] [CrossRef]
- Manimaran, N.; Rajendran, S.; Manivannan, M.; John Mary, S. Corrosion inhibition of carbon steel by polyacrylamide. Res. J. Chem. Sci. 2012, 2, 52–57. [Google Scholar]
- Takashi, O.; Takahisa, S.; Noboru, S.; Günter, P.; Helmut, S.; Otto, W.; Klaus, M.; Helmut, G. Acrylic Acid and Derivatives. 2020. Available online: https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_161.pub4 (accessed on 3 January 2020).
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Duran, B.; Bereket, G.; Duran, M. Electrochemical synthesis and characterization of poly (m-phenylenediamine) films on copper for corrosion protection. Prog. Org. Coat. 2012, 73, 162–168. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.; Comins, J. Corrosion of copper in aerated acidic pickling solutions and its inhibition by 3-amino1,2,4-triazole-5-thiol. J. Colloid Interface Sci. 2007, 306, 96–104. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K. Copper corrosion inhibition in O2-Saturated H2SO4 solutions. Corros. Sci. 2010, 52, 1194–1204. [Google Scholar] [CrossRef]
- Davis, J.R. Copper and Copper Alloys; ASM International: Hillsboro, OR, USA, 2001. [Google Scholar]
- Del PB Hernández, R.; Aoki, I.; Tribollet, B.; De Melo, H. Electrochemical impedance spectroscopy investigation of the electrochemical behaviour of copper coated with artificial patina layers and submitted to wet and dry cycles. Electrochim. Acta 2011, 56, 2801–2814. [Google Scholar] [CrossRef]
- Deyab, M.A.; Essehli, R.; El Bali, B. Inhibition of copper corrosion in cooling seawater under flowing conditions by novel pyrophosphate. RSC Adv. 2015, 5, 64326–64334. [Google Scholar] [CrossRef]
- Alhumade, H.; Abdala, A.; Yu, A.; Elkamel, A.; Simon, L. Corrosion inhibition of copper in sodium chloride solution using polyetherimide/graphene composites. Can. J. Chem. Eng. 2016, 94, 896–904. [Google Scholar] [CrossRef]
- Souto, R.; Sánchez, M.P.; Barrera, M.; González, S.; Salvarezza, R.; Arvia, A. The kinetics of pitting corrosion of copper in alkaline solutions containing sodium perchlorate. Electrochim. Acta 1992, 37, 1437–1443. [Google Scholar] [CrossRef]
- Habib, K. In-situ monitoring of pitting corrosion of copper alloys by holographic interferometry. Corros. Sci. 1998, 40, 1435–1440. [Google Scholar] [CrossRef]
- El-Mahdy, G.A.; Kim, K.B. AC impedance study on the atmospheric corrosion of aluminum under periodic wet–dry conditions. Electrochim. Acta 2004, 49, 1937–1948. [Google Scholar] [CrossRef]
- Attia, A.A.; ElMelegy, E.M.; El-Batouti, M.; Ahmed, A.-M.M. Anodic Corrosion Inhibition in Presence of Protic Solvents. Asian J. Chem. 2016, 28, 267–272. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Abd-El-Nabey, B.; Khamis, E.; Abd-El-Khalek, D. A natural extract as scale and corrosion inhibitor for steel surface in brine solution. Desalination 2011, 278, 337–342. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Wang, F. Experimental and theoretical studies for corrosion inhibition of carbon steel by imidazoline derivative in 5% NaCl saturated Ca(OH)2 solution. Electrochim. Acta 2011, 58, 427–436. [Google Scholar] [CrossRef]
- Mihajlovic, M.B.P.; Antonijevic, M.M. Copper corrosion inhibitors. Period 2008–2014. A review. Int. J. Electrochem. Sci. 2015, 10, 1027–1053. [Google Scholar]
- Antonijevic, M.; Petrovic, M. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Khaled, K.F. Corrosion control of copper in nitric acid solutions using some amino acids—A combined experimental and theoretical study. Corros. Sci. 2010, 52, 3225–3234. [Google Scholar] [CrossRef]
- Barouni, K.; Bazzi, L.; Salghi, R.; Mihit, M.; Hammouti, B.; Albourine, A.; El Issami, S. Some amino acids as corrosion inhibitors for copper in nitric acid solution. Mater. Lett. 2008, 62, 3325–3327. [Google Scholar] [CrossRef]
- Toghan, A.; Dardeer, H.M.; Gadow, H.S.; Elabbasy, H.M. New promising halogenated cyclic imides derivatives as Potential Corrosion Inhibitors for Carbon Steel in Acidic Environment. J. Mol. Liq. 2021, 325, 115136. [Google Scholar] [CrossRef]
- Manjula, P.; Manonmani, S.; Jayaram, P.; Rajendran, S. Corrosion behaviour of carbon steel in the presence of N-cetyl-N,N,N-trimethylammonium bromide, Zn2+ and calcium gluconate.Anti-Corros. Methods Mater. 2001, 48, 319–324. [Google Scholar]
- Erbil, M. The determination of corrosion rates by analysis of AC impedance diagrams. Chim. Acta Turc. 1988, 1, 59–70. [Google Scholar]
- Touhami, F.; Aouniti, A.; Abed, Y.; Hammouti, B.; Kertit, S.; Ramdani, A.; Elkacemi, K. Corrosion inhibition of armco iron in 1 M HCl media by new bipyrazolic derivatives. Corros. Sci. 2000, 42, 929–940. [Google Scholar] [CrossRef]
- Christov, M.; Popova, A. Adsorption characteristics of corrosion inhibitors from corrosion rate measurements. Corros. Sci. 2004, 46, 1613–1620. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M. Cefotaxime sodium: A new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1007–1011. [Google Scholar] [CrossRef]
- Okafor, P.C.; Zheng, Y. Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]
- Zhao, T.; Mu, G. The adsorption and corrosion inhibition of anion surfactants on aluminium surface in hydrochloric acid. Corros. Sci. 1999, 41, 1937–1944. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Lagrenee, M. The substituted 1,3,4-oxadiazoles: A new class of corrosion inhibitors of mild steel in acidic media. Corros. Sci. 2000, 42, 127–146. [Google Scholar] [CrossRef]
- Durnie, W.; De Marco, R.; Jefferson, A.; Kinsella, B. Development of a Structure-Activity Relationship for Oil Field Corrosion Inhibitors. J. Electrochem. Soc. 1999, 146, 1751–1756. [Google Scholar] [CrossRef]
- ElAchouri, M.; Hajji, M.S.; Salem, M.; Kertit, S.; Aride, J.; Coudert, R.; Essassi, E. Some Nonionic Surfactants as Inhibitors of the Corrosion of Iron in Acid Chloride Solutions. Corrosion 1996, 52, 103–108. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Yin, X.; Yang, W.; Chen, Y. Experimental and theoretical study of corrosion inhibition of 3-pyridinecarbozalde thiosemicarbazone for mild steel in hydrochloric acid. Corros. Sci. 2013, 74, 206–213. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry 2B; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Marsh, J. Advanced Organic Chemistry, 3rd ed.; Wiley: Eastern New Delhi, India, 1988. [Google Scholar]
- Eddy, N.O.; Patricia, A.E.; Mamza, P.A.P. Ethanol extract of Terminalia catappa as a green inhibitor for the corrosion of mild steel in H2SO4. Green Chem. Lett. Rev. 2009, 2, 223–231. [Google Scholar] [CrossRef]
- Noor, E. The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros. Sci. 2005, 47, 33–55. [Google Scholar] [CrossRef]
- Aoun, S.B. Highly Efficient Corrosion Inhibition of Carbon Steel in Aggressive Acidic Media with a Pyridazinium-based Ionic Liquid. Int. J. Electrochem. Sci. 2013, 8, 10788–10804. [Google Scholar]
- Muller, U. Inorganic Structure Chemistry; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2006; Volume 1, pp. 73–81. [Google Scholar]
- Trabanelli, G.; Carassiti, V. Advances in Corrosion Science and Technology; Plenum Press: New York, NY, USA, 1970; Volume 1, pp. 147–228. [Google Scholar]
- Sayed, S.Y.; El-Deab, M.S.; El-Anadouli, B.E.; Ateya, B.G. Synergistic Effects of Benzotriazole and Copper Ions on the Electrochemical Impedance Spectroscopy and Corrosion Behavior of Iron in Sulfuric Acid. J. Phys. Chem. B 2003, 107, 5575–5585. [Google Scholar] [CrossRef]
- Assad, H.; Kumar, A. Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. J. Mol. Liq. 2021, 344, 117755. [Google Scholar] [CrossRef]
- Farag, A.A.; Abdallah, H.E.; Badr, E.A.; Mohamed, E.A.; Ali, A.I.; El-Etre, A. The inhibition performance of morpholinium derivatives on corrosion behavior of carbon steel in the acidized formation water: Theoretical, experimental and biocidal evaluations. J. Mol. Liq. 2021, 341, 117348. [Google Scholar] [CrossRef]
- Hashem, H.E.; Mohamed, E.A.; Farag, A.A.; Negm, N.A.; Azmy, E.A.M. New heterocyclic Schiff base-metal complex: Synthesis, characterization, density functional theory study, and antimicrobial evaluation. Appl. Organomet. Chem. 2021, 35, e6322. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Hashem, H.E.; Azmy, E.M.; Negm, N.A.; Farag, A.A. Synthesis, structural analysis, and inhibition approach of novel eco-friendly chalcone derivatives on API X65 steel corrosion in acidic media assessment with DFT & MD studies. Environ. Technol. Innov. 2021, 24, 101966. [Google Scholar] [CrossRef]
- Shaban, S.M.; a Badr, E.; Shenashen, M.; Farag, A. Fabrication and characterization of encapsulated Gemini cationic surfactant as anticorrosion material for carbon steel protection in down-hole pipelines. Environ. Technol. Innov. 2021, 23, 101603. [Google Scholar] [CrossRef]
- Hashem, H.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M. Experimental and theoretical assessment of benzopyran compounds as inhibitors to steel corrosion in aggressive acid solution. J. Mol. Struct. 2021, 1249, 131641. [Google Scholar] [CrossRef]
- Farag, A. Oil-in-water emulsion of a heterocyclic adduct as a novel inhibitor of API X52 steel corrosion in acidic solution. Corros. Rev. 2018, 36, 575–588. [Google Scholar] [CrossRef]
- Farag, A.A.; Eid, A.; Shaban, M.; Mohamed, E.A.; Raju, G. Integrated modeling, surface, electrochemical, and biocidal investigations of novel benzothiazoles as corrosion inhibitors for shale formation well stimulation. J. Mol. Liq. 2021, 336, 116315. [Google Scholar] [CrossRef]
- Farag, A.A.; Badr, E.A. Non-ionic surfactant loaded on gel capsules to protect downhole tubes from produced water in acidizing oil wells. Corros. Rev. 2020, 38, 151–164. [Google Scholar] [CrossRef]
- Anwer, K.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M.; Sayed, G.H. Corrosion inhibition performance and computational studies of pyridine and pyran derivatives for API X-65 steel in 6 M H2SO4. J. Ind. Eng. Chem. 2021, 97, 523–538. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Farag, A.A.; Badran, B.M. Corrosion inhibitiob of mild steel using emulsified thiazole adduct in Different binder systems. Eurasian Chem.-Technol. J. 2008, 10, 67–77. [Google Scholar]
- Konno, Y.; Farag, A.A.; Tsuji, E.; Aoki, Y.; Habazaki, H. Formation of Porous Anodic Films on Carbon Steels and Their Application to Corrosion Protection Composite Coatings Formed with Polypyrrole. J. Electrochem. Soc. 2016, 163, C386–C393. [Google Scholar] [CrossRef] [Green Version]
- Shaban, M.M.; Negm, N.; Farag, R.; Fadda, A.; Gomaa, A.E.; Farag, A.; Migahed, M. Anti-corrosion, antiscalant and anti-microbial performance of some synthesized trimeric cationic imidazolium salts in oilfield applications. J. Mol. Liq. 2022, 351, 118610. [Google Scholar] [CrossRef]
- Farag, A.A.; Mohamed, E.A.; Sayed, G.H.; Anwer, K.E. Experimental/computational assessments of API steel in 6 M H2SO4 medium containing novel pyridine derivatives as corrosion inhibitors. J. Mol. Liq. 2021, 330, 115705. [Google Scholar] [CrossRef]
- Turnbull, J.; Szukalo, R.; Zagidulin, D.; Biesinger, M.; Shoesmith, D. The kinetics of copper corrosion in nitric acid. Mater. Corros. 2020, 72, 348–360. [Google Scholar] [CrossRef]
- Niamien, P.M.; Kouassi, H.A.; Trokourey, A.; Essy, F.K.; Sissouma, D.; Bokra, Y. Copper Corrosion Inhibition in 1 M HNO3 by Two Benzimidazole Derivatives. Int. Sch. Res. Netw. 2012, 2012, 623754. [Google Scholar] [CrossRef] [Green Version]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE: Houston, TX, USA, 1975. [Google Scholar]
- Johnson, H.E.; Leja, J. On the Potential/pH-Diagrams of Cu–NH3–H2O and Zn–NH3–H2O systems. J. Electrochem. Soc. 1965, 112, 638–641. [Google Scholar] [CrossRef]
- Karthik, G.; Sundaravadivelu, M. Investigations of the inhibition of copper corrosion in nitric acid solutions by levetiracetam drug. Egypt. J. Pet. 2016, 25, 481–493. [Google Scholar] [CrossRef]

















| Polymer | Conc. (mg/L) | Temperature (K) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 288 | 298 | 308 | 318 | ||||||||||
| CR | % IE | θ | CR | % IE | θ | CR | % IE | θ | CR | % IE | θ | ||
| Blank | 0 | 75 | -- | -- | 98 | -- | -- | 112 | -- | -- | 123 | -- | -- |
| PAA | 50 | 36 | 58 | 0.58 | 48 | 51 | 0.51 | 60 | 46 | 0.46 | 68 | 45 | 0.45 |
| 100 | 22 | 71 | 0.71 | 37 | 62 | 0.62 | 47 | 58 | 0.58 | 57 | 54 | 0.54 | |
| 150 | 16 | 79 | 0.79 | 28 | 71 | 0.71 | 36 | 68 | 0.68 | 43 | 65 | 0.65 | |
| 200 | 12 | 84 | 0.84 | 23 | 77 | 0.77 | 30 | 73 | 0.73 | 37 | 70 | 0.70 | |
| 250 | 11 | 85 | 0.85 | 20 | 80 | 0.80 | 26 | 77 | 0.77 | 34 | 72 | 0.72 | |
| PAM | 50 | 23 | 69 | 0.69 | 36 | 63 | 0.63 | 49 | 56 | 0.56 | 63 | 49 | 0.49 |
| 100 | 15 | 80 | 0.80 | 28 | 71 | 0.71 | 38 | 66 | 0.66 | 48 | 61 | 0.61 | |
| 150 | 10 | 87 | 0.87 | 24 | 76 | 0.76 | 30 | 73 | 0.73 | 37 | 70 | 0.70 | |
| 200 | 7 | 91 | 0.91 | 20 | 80 | 0.80 | 24 | 79 | 0.79 | 31 | 75 | 0.75 | |
| 250 | 5 | 93 | 0.93 | 16 | 84 | 0.84 | 22 | 80 | 0.80 | 27 | 78 | 0.78 | |
| Polymer | Temp. (K) | 10−3 Kads L mol−1 | kJ mol−1 | kJ mol−1 | J mol−1 K−1 |
|---|---|---|---|---|---|
| PAA | 288 | 1.69 | −27.42 | −13.42 | 48.61 |
| 298 | 1.22 | −27.56 | 47.45 | ||
| 308 | 1.02 | −28.03 | 47.44 | ||
| 318 | 0.92 | −28.66 | 47.92 | ||
| PAM | 288 | 2.48 | −28.33 | −17.16 | 38.78 |
| 298 | 1.88 | −28.63 | 38.49 | ||
| 308 | 1.51 | −29.03 | 38.54 | ||
| 318 | 1.26 | −29.49 | 38.77 |
| Polymer | Drug Conc. (mg/L) | Ea* kJ mol−1 | ∆H* kJ mol−1 | ∆S* J mol−1 K−1 |
|---|---|---|---|---|
| Blank | 0 | 12.39 | 9.81 | −3.74 |
| PAA | 50 | 16.38 | 13.72 | 3.74 |
| 100 | 19.12 | 19.04 | 19.13 | |
| 150 | 21.12 | 22.70 | 28.27 | |
| 200 | 23.53 | 26.11 | 37.83 | |
| 250 | 28.60 | 30.76 | 51.55 | |
| PAM | 50 | 25.44 | 22.86 | 32.01 |
| 100 | 27.43 | 24.94 | 36.17 | |
| 150 | 30.76 | 28.85 | 46.56 | |
| 200 | 34.92 | 33.26 | 58.62 | |
| 250 | 41.32 | 40.41 | 80.24 |
| Polymer Conc. (mg/L) | PAA | PAM | ||
|---|---|---|---|---|
| 103 k1, h−1 | t1/2, h | 103 k1, h−1 | t1/2, h | |
| Blank | 282 | 2.46 | 282 | 2.46 |
| 50 | 131 | 5.29 | 129 | 5.37 |
| 100 | 100 | 6.93 | 112 | 6.19 |
| 150 | 90 | 7.70 | 86 | 8.06 |
| 200 | 82 | 8.451 | 80 | 8.66 |
| 250 | 62 | 11.18 | 65 | 10.66 |
| Polymer | Conc. (mg/L) | Ecorr (mV(SCE)) | βa (mV/dec.) | −βc (mV/dec.) | icorr (µA/cm2) | Rp (ohm cm2) | % IE | θ |
|---|---|---|---|---|---|---|---|---|
| Blank | 0 | 22 | 56 | 121 | 211 | 79 | -- | -- |
| PAA | 50 | 24 | 50 | 111 | 95 | 158 | 55 | 0.55 |
| 100 | 24 | 52 | 96 | 74 | 200 | 65 | 0.65 | |
| 150 | 27 | 47 | 94 | 57 | 239 | 73 | 0.73 | |
| 200 | 36 | 45 | 91 | 44 | 298 | 79 | 0.79 | |
| 250 | 28 | 42 | 96 | 40 | 318 | 81 | 0.81 | |
| PAM | 50 | 31 | 46 | 97 | 89 | 152 | 58 | 0.58 |
| 100 | 37 | 49 | 99 | 65 | 219 | 69 | 0.69 | |
| 150 | 32 | 38 | 95 | 49 | 241 | 77 | 0.77 | |
| 200 | 42 | 45 | 102 | 38 | 357 | 82 | 0.82 | |
| 250 | 48 | 55 | 106 | 32 | 492 | 85 | 0.85 |
| Polymer | Conc. (mg/L) | Rs (ohm cm2) | Rct (ohm cm2) | CPE (µF/cm2) | % IE | θ |
|---|---|---|---|---|---|---|
| Blank | 0 | 1.2 | 55 | 288 | -- | -- |
| PAA | 50 | 1.3 | 141 | 112 | 61 | 0.61 |
| 100 | 1.7 | 190 | 98 | 71 | 0.71 | |
| 150 | 1.9 | 250 | 83 | 78 | 0.78 | |
| 200 | 2.7 | 305 | 66 | 82 | 0.82 | |
| 250 | 1.5 | 344 | 57 | 84 | 0.84 | |
| PAM | 50 | 1.8 | 138 | 113 | 60 | 0.60 |
| 100 | 1.6 | 204 | 95 | 73 | 0.73 | |
| 150 | 2.1 | 262 | 79 | 79 | 0.79 | |
| 200 | 1.5 | 367 | 54 | 85 | 0.85 | |
| 250 | 1.7 | 458 | 43 | 88 | 0.88 |
| Parameters | PAA | PAM |
|---|---|---|
| EHOMO | −0.2645 | −0.2708 |
| ELUMO | −0.0527 | −0.0718 |
| ΔEL-H | 0.2118 | 0.1990 |
| I | 0.2645 | 0.2708 |
| A | 0.0527 | 0.0718 |
| χ | 0.1586 | 0.1713 |
| η | 0.1059 | 0.0995 |
| σ | 9.4429 | 10.0503 |
| ΔN | 20.6520 | 22.0126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toghan, A.; Fawzy, A.; Al Bahir, A.; Alqarni, N.; Sanad, M.M.S.; Khairy, M.; Alakhras, A.I.; Farag, A.A. Computational Foretelling and Experimental Implementation of the Performance of Polyacrylic Acid and Polyacrylamide Polymers as Eco-Friendly Corrosion Inhibitors for Copper in Nitric Acid. Polymers 2022, 14, 4802. https://doi.org/10.3390/polym14224802
Toghan A, Fawzy A, Al Bahir A, Alqarni N, Sanad MMS, Khairy M, Alakhras AI, Farag AA. Computational Foretelling and Experimental Implementation of the Performance of Polyacrylic Acid and Polyacrylamide Polymers as Eco-Friendly Corrosion Inhibitors for Copper in Nitric Acid. Polymers. 2022; 14(22):4802. https://doi.org/10.3390/polym14224802
Chicago/Turabian StyleToghan, Arafat, Ahmed Fawzy, Areej Al Bahir, Nada Alqarni, Moustafa M. S. Sanad, Mohamed Khairy, Abbas I. Alakhras, and Ahmed A. Farag. 2022. "Computational Foretelling and Experimental Implementation of the Performance of Polyacrylic Acid and Polyacrylamide Polymers as Eco-Friendly Corrosion Inhibitors for Copper in Nitric Acid" Polymers 14, no. 22: 4802. https://doi.org/10.3390/polym14224802
APA StyleToghan, A., Fawzy, A., Al Bahir, A., Alqarni, N., Sanad, M. M. S., Khairy, M., Alakhras, A. I., & Farag, A. A. (2022). Computational Foretelling and Experimental Implementation of the Performance of Polyacrylic Acid and Polyacrylamide Polymers as Eco-Friendly Corrosion Inhibitors for Copper in Nitric Acid. Polymers, 14(22), 4802. https://doi.org/10.3390/polym14224802