Silk Fibroin Hydrogels Incorporated with the Antioxidant Extract of Stryphnodendron adstringens Bark
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
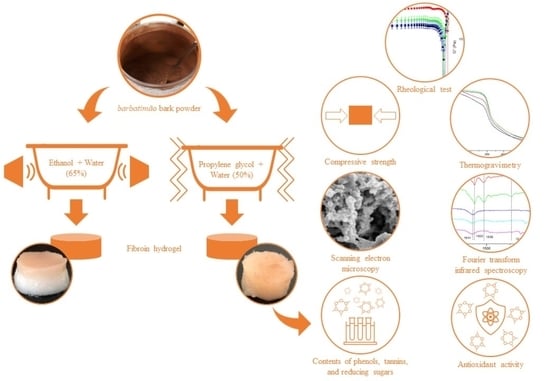
2.2. Barbatimão Bark Preparation
2.3. Obtaining Barbatimão Extracts
2.4. Extract Characterization
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Tannins Content (TTC)
2.4.3. Ferric Reducing Ability Power (FRAP)
2.4.4. Oxygen Radical Absorbance Capacity (ORAC)
2.4.5. Total Reducing Sugar Content (TRSC)
2.5. Preparation of Silk Fibroin Aqueous Solution
2.6. Incorporation of the Extracts into the Silk Fibroin Hydrogel
2.7. Hydrogel Characterization
2.7.1. Scanning Electron Microscopy (SEM)
2.7.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.7.3. Thermogravimetry Analysis (TGA)
2.7.4. Compressive Strength
2.7.5. Rheological Tests
2.8. Statistical Analyses
3. Results and Discussion
3.1. Extract Characterization
3.2. Hydrogel Characterization
3.3. Scanning Electron Microscopy (SEM)
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Thermogravimetry (TGA)
3.6. Compressive Strength
3.7. Rheological Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal Plants in Brazil: Pharmacological Studies, Drug Discovery, Challenges and Perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef] [PubMed]
- de Souza Ribeiro, M.M.; dos Santos, L.C.; de Novais, N.S.; Viganó, J.; Veggi, P.C. An Evaluative Review on Stryphnodendron Adstringens Extract Composition: Current and Future Perspectives on Extraction and Application. Ind. Crops Prod. 2022, 187, 115325. [Google Scholar] [CrossRef]
- Holetz, F.B.; Ueda-Nakamura, T.; Dias Filho, B.P.; Mello, J.C.P.D.; Morgado-Díaz, J.A.; Toledo, C.E.M.D.; Nakamura, C.V. Biological Effects of Extracts Obtained from Stryphnodendron Adstringens on Herpetomonas Samuelpessoai. Mem. Inst. Oswaldo Cruz. 2005, 100, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, K.; Rozental, S.; de Mello, J.C.P.; Nakamura, C.V. Activity of Tannins from Stryphnodendron Adstringens on Cryptococcus Neoformans: Effects on Growth, Capsule Size and Pigmentation. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza-Moreira, T.M.; Queiroz-Fernandes, G.M.; Pietro, R.C.L.R. Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report. Molecules 2018, 23, 910. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, E.L.; da Silva Baldivia, D.; Leite, D.F.; de Castro, D.T.H.; Campos, J.F.; de Oliveira, C.F.R.; de Carvalho, J.T.G.; dos Santos, U.P.; de Picoli Souza, K. Antioxidant and Anticancer Activities from Stryphnodendron Adstringens. Free Radic. Biol. Med. 2018, 128, S66. [Google Scholar] [CrossRef]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Souza, P.M.; Elias, S.T.; Simeoni, L.A.; de Paula, J.E.; Gomes, S.M.; Guerra, E.N.S.; Fonseca, Y.M.; Silva, E.C.; Silveira, D.; Magalhães, P.O. Plants from Brazilian Cerrado with Potent Tyrosinase Inhibitory Activity. PLoS ONE 2012, 7, e48589. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.G.; Nascimento, A.M.; Henriques, B.O.; Chávez-Fumagalli, M.A.; Franca, J.R.; Duarte, M.C.; Lage, P.S.; Andrade, P.H.R.; Lage, D.P.; Rodrigues, L.B.; et al. Antileishmanial Activity of Standardized Fractions of Stryphnodendron Obovatum (Barbatimão) Extract and Constituent Compounds. J. Ethnopharmacol. 2015, 165, 238–242. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of Ultrasound Assisted Extraction of Antioxidant Compounds from Marjoram (Origanum Majorana L.) Using Response Surface Methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef]
- Hadi, A.; Nawab, A.; Alam, F.; Zehra, K. Alginate/Aloe Vera Films Reinforced with Tragacanth Gum. Food Chem. Mol. Sci. 2022, 4, 100105. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.Y.; Yeo, J.H.; Lee, K.G.; Lee, Y.W.; Park, Y.H.; Nahm, J.H.; Cho, C.S. Effects of Poloxamer on the Gelation of Silk Sericin. Macromol. Rapid Commun. 2000, 21, 1302–1305. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk Fibroin as a Vehicle for Drug Delivery Applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for Delivery of Bioactive Agents: A Historical Perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Inpanya, P.; Faikrua, A.; Ounaroon, A.; Sittichokechaiwut, A.; Viyoch, J. Effects of the Blended Fibroin/Aloe Gel Film on Wound Healing in Streptozotocin-Induced Diabetic Rats. Biomed. Mater. 2012, 7, 035008. [Google Scholar] [CrossRef]
- Kim, D.K.; Sim, B.R.; Khang, G. Nature-Derived Aloe Vera Gel Blended Silk Fibroin Film Scaffolds for Cornea Endothelial Cell Regeneration and Transplantation. ACS Appl. Mater. Interfaces 2016, 8, 15160–15168. [Google Scholar] [CrossRef]
- Li, C.; Luo, T.; Zheng, Z.; Murphy, A.R.; Wang, X.; Kaplan, D.L. Curcumin-Functionalized Silk Materials for Enhancing Adipogenic Differentiation of Bone Marrow-Derived Human Mesenchymal Stem Cells Chunmei. Acta Biomater. 2015, 11, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Mori, C.L.S.; dos Passos, N.A.; Oliveira, J.E.; Mattoso, L.H.C.; Mori, F.A.; Carvalho, A.G.; de Souza Fonseca, A.; Tonoli, G.H.D. Electrospinning of Zein/Tannin Bio-Nanofibers. Ind. Crops Prod. 2014, 52, 298–304. [Google Scholar] [CrossRef]
- Nascimento, K.M.; Cavalheiro, J.B.; Netto, A.Á.M.; da Silva Scapim, M.R.; de Cássia Bergamasco, R. Properties of Alginate Films Incorporated with Free and Microencapsulated Stryphnodendron Adstringens Extract (Barbatimão). Food Packag. Shelf Life 2021, 28, 100637. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A Critical Analysis of Extraction Techniques Used for Botanicals: Trends, Priorities, Industrial Uses and Optimization Strategies. TrAC—Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Sousa, J.N.; Pedroso, N.B.; Borges, L.L.; Oliveira, G.A.R.; Paula, J.R.; Conceicao, E.C. Optimization of Ultrasound-Assisted Extraction of Polyphenols, Tannins and Epigallocatechin Gallate from Barks of Stryphnodendron Adstringens (Mart.) Coville Bark Extracts. Pharmacogn. Mag. 2014, 10, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baurin, N.; Arnoult, E.; Scior, T.; Do, Q.T.; Bernard, P. Preliminary Screening of Some Tropical Plants for Anti-Tyrosinase Activity. J. Ethnopharmacol. 2002, 82, 155–158. [Google Scholar] [CrossRef]
- Campos, P.; Yariwake, J.H.; Lanças, F.M. Effect of X- and Gamma-Rays on Phenolic Compounds from Maytenus Aquifolium Martius. J. Radioanal Nucl. Chem. 2005, 264, 707–709. [Google Scholar] [CrossRef]
- ANVISA Farmacopeia Brasileira: Plantas Medicinais, 6th ed.; ANVISA: Brasília, Brazil, 2019.
- Viganó, J.; Assis, B.F.d.P.; Náthia-Neves, G.; dos Santos, P.; Meireles, M.A.A.; Veggi, P.C.; Martínez, J. Extraction of Bioactive Compounds from Defatted Passion Fruit Bagasse (Passiflora edulis Sp.) Applying Pressurized Liquids Assisted by Ultrasound. Ultrason. Sonochem. 2020, 64, 104999. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of Total Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe: First Action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Nogueira, G.M.; de Moraes, M.A.; Rodas, A.C.D.; Higa, O.Z.; Beppu, M.M. Hydrogels from Silk Fibroin Metastable Solution: Formation and Characterization from a Biomaterial Perspective. Mater. Sci. Eng. C 2011, 31, 997–1001. [Google Scholar] [CrossRef]
- Jauregi, P.; Guo, Y.; Adeloye, J.B. Whey Proteins-Polyphenols Interactions Can Be Exploited to Reduce Astringency or Increase Solubility and Stability of Bioactives in Foods. Food Res. Int. 2021, 141, 110019. [Google Scholar] [CrossRef]
- Jauregi, P.; Olatujoye, J.B.; Cabezudo, I.; Frazier, R.A.; Gordon, M.H. Astringency Reduction in Red Wine by Whey Proteins. Food Chem. 2016, 199, 547–555. [Google Scholar] [CrossRef]
- Oliveira, A.; Amaro, A.L.; Pintado, M. Impact of Food Matrix Components on Nutritional and Functional Properties of Fruit-Based Products. Curr. Opin. Food Sci. 2018, 22, 153–159. [Google Scholar] [CrossRef]
- Li, N.; Girard, A.L. Impact of PH and Temperature on Whey Protein-Proanthocyanidin Interactions and Foaming Properties. Food Hydrocoll. 2023, 134, 108100. [Google Scholar] [CrossRef]
- Ardisson, L.; Godoy, J.S.; Ferreira, L.A.M.; Stehmann, J.R.; Brandão, M.G.L. Preparação e Caracterização de Extratos Glicólicos Enriquecidos Em Taninos a Partir Das Cascas de Stryphnodendron Adstringens (Mart.) Coville (Barbatimão). Rev. Bras. De Farmacogn. 2002, 12, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.C.; Costa, W.F.; Batista, F.; Santos, L.R.; Ferri, P.H.; Ferreira, H.D.; Seraphin, J.C. Seasonal Variation in the Content of Tannins in Barks of Barbatimão Species. Rev. Bras. De Farmacogn. 2006, 16, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food. Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, L.; Goetze, D.; Ribeiro, C.T.; Paludo, N.; Rodrigues, E.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Identification of Bioactive Compounds from Vitis Labrusca L. Variety Concord Grape Juice Treated With Commercial Enzymes: Improved Yield and Quality Parameters. Food Bioproc. Tech. 2016, 9, 365–377. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Joo Kim, H.; Wada, M.; Kaplan, D.L. Three-Dimensional Aqueous-Derived Biomaterial Scaffolds from Silk Fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of Silk Fibroin Sol-Gel Transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef]
- Jing, J.; Liang, S.; Yan, Y.; Tian, X.; Li, X. Fabrication of Hybrid Hydrogels from Silk Fibroin and Tannic Acid with Enhanced Gelation and Antibacterial Activities. ACS Biomater. Sci. Eng. 2019, 5, 4601–4611. [Google Scholar] [CrossRef]
- Ribeiro, M.; de Moraes, M.A.; Beppu, M.M.; Monteiro, F.J.; Ferraz, M.P. The Role of Dialysis and Freezing on Structural Conformation, Thermal Properties and Morphology of Silk Fibroin Hydrogels. Biomatter 2014, 4, e28536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusa, C.C.; Bridges, C.; Ha, S.W.; Tonelli, A.E. Conformational Changes Induced in Bombyx Mori Silk Fibroin by Cyclodextrin Inclusion Complexation. Macromolecules 2005, 38, 5640–5646. [Google Scholar] [CrossRef]
- Guziewicz, N.; Best, A.; Perez-Ramirez, B.; Kaplan, D.L. Lyophilized Silk Fibroin Hydrogels for the Sustained Local Delivery of Therapeutic Monoclonal Antibodies. Biomaterials 2011, 32, 2642–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Knight, D.P.; Shao, Z.; Vollrath, F. Regenerated Bombyx Silk Solutions Studied with Rheometry and FTIR. Polymer 2001, 42, 09969–09974. [Google Scholar] [CrossRef]
- Lissner, H.; Wehrer, M.; Reinicke, M.; Horváth, N.; Totsche, K.U. Constraints of Propylene Glycol Degradation at Low Temperatures and Saturated Flow Conditions. Environ. Sci. Pollut. Res. 2015, 22, 3158–3174. [Google Scholar] [CrossRef]
- Huang, D.; Peng, Z.; Hu, Z.; Zhang, S.; He, J.; Cao, L.; Zhou, Y.; Zhao, F. A New Consolidation System for Aged Silk Fabrics: Effect of Reactive Epoxide-Ethylene Glycol Diglycidyl Ether. React. Funct. Polym. 2013, 73, 168–174. [Google Scholar] [CrossRef]
- Ramirez, S.M.V.; de Moraes, M.A.; Beppu, M.M. Assessing the Influence of Silkworm Cocoon’s Age on the Physicochemical Properties of Silk Fibroin-Based Materials. J. Mater. Res. 2019, 34, 1944–1949. [Google Scholar] [CrossRef]
- de Moraes, M.A.; Mahl, C.R.A.; Silva, M.F.; Beppu, M.M. Formation of Silk Fibroin Hydrogel and Evaluation of Its Drug Release Profile. J. Appl. Polym. Sci. 2015, 132, 41802. [Google Scholar] [CrossRef]
- Tamada, Y. New Process to Form a Silk Fibroin Porous 3-D Structure. Biomacromolecules 2005, 6, 3100–3106. [Google Scholar] [CrossRef]
- Brummer, R. Rheology Essentials of Cosmetics and Food Emulsions, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Ma, M.; Dong, S.; Hussain, M.; Zhou, W. Effects of Addition of Condensed Tannin on the Structure and Properties of Silk Fibroin Film. Polym. Int. 2017, 66, 151–159. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A Dual Synergistic of Curcumin and Gelatin on Thermal-Responsive Hydrogel Based on Chitosan-P123 in Wound Healing Application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]







| Nomenclature | Description |
|---|---|
| ET hydrogel | Silk fibroin hydrogel containing ethanol solution at 65% (v/v) |
| PG hydrogel | Silk fibroin hydrogel containing propylene glycol solution at 50% (v/v) |
| BT/ET hydrogel | Silk fibroin hydrogel containing the barbatimão ethanolic extract |
| BT/PG hydrogel | Silk fibroin hydrogel containing the barbatimão propylene glycol extract |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Brito, V.P.; de Souza Ribeiro, M.M.; Viganó, J.; de Moraes, M.A.; Veggi, P.C. Silk Fibroin Hydrogels Incorporated with the Antioxidant Extract of Stryphnodendron adstringens Bark. Polymers 2022, 14, 4806. https://doi.org/10.3390/polym14224806
de Brito VP, de Souza Ribeiro MM, Viganó J, de Moraes MA, Veggi PC. Silk Fibroin Hydrogels Incorporated with the Antioxidant Extract of Stryphnodendron adstringens Bark. Polymers. 2022; 14(22):4806. https://doi.org/10.3390/polym14224806
Chicago/Turabian Stylede Brito, Vivian P., Maurício M. de Souza Ribeiro, Juliane Viganó, Mariana A. de Moraes, and Priscilla C. Veggi. 2022. "Silk Fibroin Hydrogels Incorporated with the Antioxidant Extract of Stryphnodendron adstringens Bark" Polymers 14, no. 22: 4806. https://doi.org/10.3390/polym14224806
APA Stylede Brito, V. P., de Souza Ribeiro, M. M., Viganó, J., de Moraes, M. A., & Veggi, P. C. (2022). Silk Fibroin Hydrogels Incorporated with the Antioxidant Extract of Stryphnodendron adstringens Bark. Polymers, 14(22), 4806. https://doi.org/10.3390/polym14224806