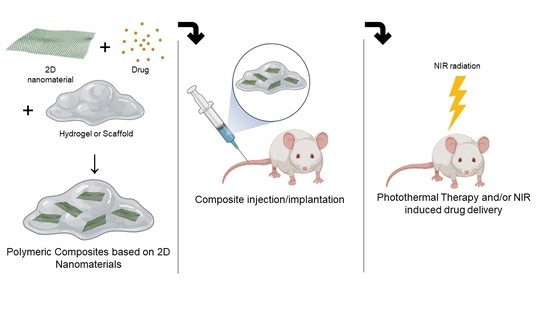
New Polymeric Composites Based on Two-Dimensional Nanomaterials for Biomedical Applications
Abstract
:1. Introduction

1.1. Examples of Biomedical Applications of 2DnMat
1.2. The Current State of 2DnMat
2. 2D Nanomaterials Polymeric Composites for Biomedical Applications
2.1. Black Phosphorus
2.1.1. PTT
2.1.2. Drug Delivery
2.1.3. Wound Healing
2.1.4. Tissue Engineering
2.2. TMDs
2.3. MXenes
2.3.1. In Vitro Studies of Mxene-Based Polymeric Composites
2.3.2. PTT
2.3.3. Drug Delivery
2.3.4. Wound Healing
2.3.5. Biosensing
2.3.6. Gas Therapy
2.4. Other 2D Nanomaterials
2.5. Potential Clinical Use
3. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-dimensional materials in biomedical, biosensing and sensing applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef] [PubMed]
- Anju, S.; Mohanan, P.V. Biomedical applications of transition metal dichalcogenides (TMDCs). Synth. Met. 2021, 271, 116610. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Zhou, L.; Li, J.; Chen, H. 2D nanostructures beyond graphene: Preparation, biocompatibility and biodegradation behaviors. J. Mater. Chem. B 2020, 8, 2974–2989. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Qu, K.Y.; Zhang, X.F.; Huang, N.P. Recent Advances in the Application of Two-Dimensional Nanomaterials for Neural Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 3503–3529. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Zhao, M.; Liu, G.; Wu, J. 2D nanomaterials for tissue engineering application. Nano Res. 2020, 13, 2019–2034. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D nanomaterials for biomedical applications. Mater. Today 2021, 50, 276–302. [Google Scholar] [CrossRef]
- Samal, R.; Sanyal, G.; Chakraborty, B.; Rout, C.S. Two-dimensional transition metal phosphorous trichalcogenides (MPX3): A review on emerging trends, current state and future perspectives. J. Mater. Chem. A 2021, 9, 2560–2591. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Wang, R.; Zhang, Y.; Mahmood, A.; Ouyang, Z.; Zhang, H.; Guo, Z. Recent Developments in Emerging Two-Dimensional Materials and Their Applications; Royal Society of Chemistry: London, UK, 2020; Volume 8, ISBN 0000000272. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Flexible and low power CO gas sensor with Au-functionalized 2D WS2 nanoflakes. Sens. Actuators B Chem. 2020, 313, 128040. [Google Scholar] [CrossRef]
- Hau, H.H.; Duong, T.T.H.; Man, N.K.; Thi Viet Nga, T.; Thi Xuan, C.; Thi Thanh Le, D.; Van Toan, N.; Hung, C.M.; Van Duy, N.; Van Hieu, N.; et al. Enhanced NO2 gas-sensing performance at room temperature using exfoliated MoS2 nanosheets. Sens. Actuators A Phys. 2021, 332, 113137. [Google Scholar] [CrossRef]
- Zaheer, A.; Zahra, S.A.; Iqbal, M.Z.; Mahmood, A.; Khan, S.A.; Rizwan, S. Nickel-adsorbed two-dimensional Nb2C MXene for enhanced energy storage applications. RSC Adv. 2022, 12, 4624–4634. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Z.; Li, X.; Liu, Z.; Li, H.; Liang, G.; Wang, D.; Huang, Q.; Zhang, S.; Chen, S.; et al. A Wholly Degradable, Rechargeable Zn-Ti3C2 MXene Capacitor with Superior Anti-Self-Discharge Function. ACS Nano 2019, 13, 8275–8283. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Paštika, J.; Mazánek, V.; Melle-Franco, M.; Sofer, Z.; Gusmão, R. Cobalt Phosphorous Trisulfide as a High-Performance Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 23638–23646. [Google Scholar] [CrossRef]
- Guo, D.; Li, X.; Jiao, Y.; Yan, H.; Wu, A.; Yang, G.; Wang, Y.; Tian, C.; Fu, H. A dual-active Co-CoO heterojunction coupled with Ti3C2-MXene for highly-performance overall water splitting. Nano Res. 2022, 15, 238–247. [Google Scholar] [CrossRef]
- Zhong, F.; Ye, J.; He, T.; Zhang, L.; Wang, Z.; Li, Q.; Han, B.; Wang, P.; Wu, P.; Yu, Y.; et al. Substitutionally Doped MoSe2 for High-Performance Electronics and Optoelectronics. Small 2021, 17, 2102855. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, M.U.; Lee, C.; Yi, S.G.; Kim, M.; Choi, Y.; Cho, J.H.; Yoo, K.H. Rectifying optoelectronic memory based on WSe2/graphene heterostructures. Nanoscale Adv. 2021, 3, 4952–4960. [Google Scholar] [CrossRef]
- Jakubczak, M.; Karwowska, E.; Rozmysłowska-Wojciechowska, A.; Petrus, M.; Woźniak, J.; Mitrzak, J.; Jastrzębska, A.M. Filtration materials modified with 2d nanocomposites-a new perspective for point-of-use water treatment. Materials 2021, 14, 182. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Q.; Zhang, S.; Wu, F.; Yu, X.; Xiong, Z.; Ma, W.; Wang, D.; Zhang, X.; Xing, B. Filtration-based water treatment system embedded with black phosphorus for NIR-triggered disinfection. Environ. Sci. Nano 2019, 6, 2977–2985. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y. Two-dimensional biomaterials: Material science, biological effect and biomedical engineering applications. Chem. Soc. Rev. 2021, 50, 11381–11485. [Google Scholar] [CrossRef]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [Green Version]
- Molaei, M.J. Two-dimensional (2D) materials beyond graphene in cancer drug delivery, photothermal and photodynamic therapy, recent advances and challenges ahead: A review. J. Drug Deliv. Sci. Technol. 2021, 61, 101830. [Google Scholar] [CrossRef]
- Zhuge, F.; Zheng, Z.; Luo, P.; Lv, L.; Huang, Y.; Li, H.; Zhai, T. Nanostructured Materials and Architectures for Advanced Infrared Photodetection. Adv. Mater. Technol. 2017, 2, 1700005. [Google Scholar] [CrossRef]
- Chaves, A.; Azadani, J.G.; Alsalman, H.; da Costa, D.R.; Frisenda, R.; Chaves, A.J.; Song, S.H.; Kim, Y.D.; He, D.; Zhou, J.; et al. Bandgap engineering of two-dimensional semiconductor materials. NPJ 2D Mater. Appl. 2020, 4, 29. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicine 2021, 9, 305. [Google Scholar] [CrossRef]
- Nomura, S.; Morimoto, Y.; Tsujimoto, H.; Arake, M.; Harada, M.; Saitoh, D.; Hara, I.; Ozeki, E.; Satoh, A.; Takayama, E.; et al. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using a near-infrared absorbent. Sci. Rep. Nat. Res. 2020, 10, 9765. [Google Scholar] [CrossRef] [PubMed]
- Nanobiotechnol, J.; Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Li, L.; Wang, J. Insights into the Photothermal Conversion of 2D MXene Nanomaterials: Synthesis, Mechanism, and Applications. Adv. Funct. Mater. 2020, 30, 2000712. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Qualtieri, A.; Pisanello, F.; De Vittorio, M. Microstructure and Electrical Properties of Novel piezo-optrodes Based on Thin-Film Piezoelectric Aluminium Nitride for Sensing. IEEE Trans. Nanotechnol. 2021, 20, 10–19. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.W.; Kim, K.S. Recent trends in photoacoustic imaging techniques for 2d nanomaterial-based phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L.; Zhao, C.; Li, Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef]
- Lu, B.; Hu, S.; Wu, D.; Wu, C.; Zhu, Z.; Hu, L.; Zhang, J. Ionic liquid exfoliated Ti 3 C 2 T × MXene nanosheets for photoacoustic imaging and synergistic photothermal/chemotherapy of cancer. J. Mater. Chem. B 2022, 10, 1226–1235. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar] [CrossRef] [Green Version]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [Green Version]
- Gudz, K.Y.; Permyakova, E.S.; Matveev, A.T.; Bondarev, A.V.; Manakhov, A.M.; Sidorenko, D.A.; Filippovich, S.Y.; Brouchkov, A.V.; Golberg, D.V.; Ignatov, S.G.; et al. Pristine and Antibiotic-Loaded Nanosheets/Nanoneedles-Based Boron Nitride Films as a Promising Platform to Suppress Bacterial and Fungal Infections. ACS Appl. Mater. Interfaces 2020, 12, 42485–42498. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, R.Y.; Chen, W.; Liu, Z.; Xu, Q.; Zeng, K.; Deng, L.; Shen, L.; Liu, Y.N. A black phosphorus based synergistic antibacterial platform against drug resistant bacteria. J. Mater. Chem. B 2018, 6, 6302–6310. [Google Scholar] [CrossRef]
- Shaw, Z.L.; Kuriakose, S.; Cheeseman, S.; Dickey, M.D.; Genzer, J.; Christofferson, A.J.; Crawford, R.J.; McConville, C.F.; Chapman, J.; Truong, V.K.; et al. Antipathogenic properties and applications of low-dimensional materials. Nat. Commun. 2021, 12, 3897. [Google Scholar] [CrossRef]
- Mazinani, A.; Rastin, H.; Nine, M.J.; Lee, J.; Tikhomirova, A.; Tung, T.T.; Ghomashchi, R.; Kidd, S.; Vreugde, S.; Losic, D. Comparative antibacterial activity of 2D materials coated on porous-titania. J. Mater. Chem. B 2021, 9, 6412–6424. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; De Donato, F.; Salbini, M.; Brunetti, V.; Qualtieri, A.; Rizzi, F.; De Vittorio, M. Captive-air-bubble aerophobicity measurements of antibiofouling coatings for underwater MEMS devices. Nanomater. Nanotechnol. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Loske, L.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. 2D Nanocomposite Membranes: Water Purification and Fouling Mitigation. Membranes 2020, 10, 295. [Google Scholar] [CrossRef]
- Henriques, P.C.; Pereira, A.T.; Bogas, D.; Fernandes, J.R.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Graphene films irradiated with safe low-power NIR-emitting diodes kill multidrug resistant bacteria. Carbon N. Y. 2021, 180, 10–21. [Google Scholar] [CrossRef]
- Vasilev, K. Nanoengineered Antibacterial Coatings. Coatings 2019, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Xiao, Y.; Wang, Y.; Li, L.; Li, W.; Tao, W. Biomedical applications of 2D monoelemental materials formed by group VA and VIA: A concise review. J. Nanobiotechnol. 2021, 19, 96. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, T.; Chen, W.; Li, Y.; Wang, B. Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioact. Mater. 2020, 5, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wee, A.T.S.; Liang, Q.; Zhao, X.; Liu, M. Defect engineering of two-dimensional transition-metal dichalcogenides: Applications, challenges, and opportunities. ACS Nano 2021, 15, 2165–2181. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Mao, Y.; Li, Z. Progress and biomedical applications of MXenes. Nano Sel. 2021, 2, 1480–1508. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Thurakkal, S.; Zhang, X. Recent Advances in Chemical Functionalization of 2D Black Phosphorous Nanosheets. Adv. Sci. 2020, 7, 1902359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozmysłowska-wojciechowska, A.; Szuplewska, A.; Wojciechowski, T.; Poźniak, S.; Mitrzak, J.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A.M. Materials Science & Engineering C A simple, low-cost and green method for controlling the cytotoxicity of MXenes. Mater. Sci. Eng. C 2020, 111, 110790. [Google Scholar] [CrossRef]
- Zeng, G.; Huang, L.; Huang, Q.; Liu, M.; Xu, D.; Huang, H.; Yang, Z.; Deng, F.; Zhang, X.; Wei, Y. Rapid synthesis of MoS-PDA-Ag nanocomposites as heterogeneous catalysts and antimicrobial agents via microwave irradiation. Appl. Surf. Sci. 2018, 459, 588–595. [Google Scholar] [CrossRef]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, C.; Chen, X.; Liu, J.; Yu, Q.; Liu, Y.; Liu, J. Drug Delivery System Based on Near-Infrared Light-Responsive Molybdenum Disulfide Nanosheets Controls the High-Efficiency Release of Dexamethasone to Inhibit Inflammation and Treat Osteoarthritis. ACS Appl. Mater. Interfaces 2019, 11, 11587–11601. [Google Scholar] [CrossRef]
- Bolotsky, A.; Butler, D.; Dong, C.; Gerace, K.; Glavin, N.R.; Muratore, C.; Robinson, J.A.; Ebrahimi, A. Two-Dimensional Materials in Biosensing and Healthcare: From in Vitro Diagnostics to Optogenetics and beyond. ACS Nano 2019, 13, 9781–9810. [Google Scholar] [CrossRef] [Green Version]
- Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Vikartovska, A.; Tanvir, A.; Kasak, P.; Tkac, J. Ultrasensitive Ti3C2TX MXene/chitosan nanocomposite-based amperometric biosensor for detection of potential prostate cancer marker in urine samples. Processes 2020, 8, 580. [Google Scholar] [CrossRef]
- Yang, L.; Ma, Z.; Tian, Y.; Meng, B.; Peng, Z. Progress on self-powered wearable and implantable systems driven by nanogenerators. Micromachines 2021, 12, 666. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zeng, Q.; He, L.; Yin, P.; Sun, Y.; Hu, W.; Yang, R. Fabrication and application of biocompatible nanogenerators. iScience 2021, 24, 102274. [Google Scholar] [CrossRef]
- Salauddin, M.; Rana, S.M.S.; Sharifuzzaman, M.; Rahman, M.T.; Park, C.; Cho, H.; Maharjan, P.; Bhatta, T.; Park, J.Y. A Novel MXene Ecoflex Nanocomposite-Coated Fabric as a Highly Negative and Stable Friction Layer for High-Output Triboelectric Nanogenerators Md. Adv. Energy Mater. 2021, 11, 2002832. [Google Scholar] [CrossRef]
- Dong, Y.; Mallineni, S.S.K.; Maleski, K.; Behlow, H.; Mochalin, V.N.; Rao, A.M.; Gogotsi, Y.; Podila, R. Metallic MXenes: A new family of materials for flexible triboelectric nanogenerators. Nano Energy 2018, 44, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Cui, P.; Chen, X.; Wang, J.; Parida, K.; Lin, M.F.; Lee, P.S. Skin-touch-actuated textile-based triboelectric nanogenerator with black phosphorus for durable biomechanical energy harvesting. Nat. Commun. 2018, 9, 4280. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Lu, J.; Wan, B.; Peng, D.; Xu, Q.; Hu, G.; Peng, Y.; Pan, C.; Wang, Z.L. Piezoelectricity in Multilayer Black Phosphorus for Piezotronics and Nanogenerators. Adv. Mater. 2020, 32, 1905795. [Google Scholar] [CrossRef]
- Fabbri, R.; Saracino, E.; Treossi, E.; Zamboni, R.; Palermo, V.; Benfenati, V. Graphene glial-interfaces: Challenges and perspectives. Nanoscale 2021, 13, 4390–4407. [Google Scholar] [CrossRef] [PubMed]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-Dimensional Nanomaterials beyond Graphene for Biomedical Applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Anju, S.; Ashtami, J.; Mohanan, P.V. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater. Sci. Eng. C 2019, 97, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [Green Version]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced strategies for tissue engineering in regenerative medicine: A biofabrication and biopolymer perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef]
- Nguyen, E.P.; De Carvalho Castro Silva, C.; Merkoçi, A. Recent advancement in biomedical applications on the surface of two-dimensional materials: From biosensing to tissue engineering. Nanoscale 2020, 12, 19043–19067. [Google Scholar] [CrossRef]
- Gómez, I.J.; Alegret, N.; Dominguez-Alfaro, A.; Vázquez Sulleiro, M. Recent Advances on 2D Materials towards 3D Printing. Chemistry 2021, 3, 1314–1343. [Google Scholar] [CrossRef]
- Yang, G.; Wan, X.; Gu, Z.; Zeng, X.; Tang, J. Near infrared photothermal-responsive poly(vinyl alcohol)/black phosphorus composite hydrogels with excellent on-demand drug release capacity. J. Mater. Chem. B 2018, 6, 1622–1632. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Qiu, M.; Liang, X.; Liu, Q.; Zou, Q.; Li, Z.; Xie, Z.; Wang, D.; Dong, B.; et al. Conceptually Novel Black Phosphorus/Cellulose Hydrogels as Promising Photothermal Agents for Effective Cancer Therapy. Adv. Healthc. Mater. 2018, 7, e1701510. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef]
- Ouyang, J.; Ji, X.; Zhang, X.; Feng, C.; Tang, Z.; Kong, N.; Xie, A.; Wang, J.; Sui, X.; Deng, L.; et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc. Natl. Acad. Sci. USA 2020, 117, 28667–28677. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Y.; Yang, M.; Chang, Y.; Nie, A.; Liu, Z.; Wang, J.; Luo, Z. Black-Phosphorus-Incorporated Hydrogel as a Conductive and Biodegradable Platform for Enhancement of the Neural Differentiation of Mesenchymal Stem Cells. Adv. Funct. Mater. 2020, 30, 2000177. [Google Scholar] [CrossRef]
- Qian, Y.; Yuan, W.E.; Cheng, Y.; Yang, Y.; Qu, X.; Fan, C. Concentrically Integrative Bioassembly of a Three-Dimensional Black Phosphorus Nanoscaffold for Restoring Neurogenesis, Angiogenesis, and Immune Homeostasis. Nano Lett. 2019, 19, 8990–9001. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Li, X.; Gao, W.; Zhang, L.; Liu, J.; Zheng, Y.; Chen, H.; Shi, J. Injectable 2D MoS2-Integrated Drug Delivering Implant for Highly Efficient NIR-Triggered Synergistic Tumor Hyperthermia. Adv. Mater. 2015, 27, 7117–7122. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Jin, L.; Li, Y.; Wang, Z. Effects of Polyacrylonitrile/MoS2 Composite Nanofibers on the Growth Behavior of Bone Marrow Mesenchymal Stem Cells. ACS Appl. Nano Mater. 2018, 1, 337–343. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Y.; Deng, Q.; Jeong, S.H.; Jang, T.S.; Du, S.; Kim, H.E.; Huang, Q.; Han, C.M. Strong and biocompatible poly(lactic acid) membrane enhanced by Ti3C2Tz (MXene) nanosheets for Guided bone regeneration. Mater. Lett. 2018, 229, 114–117. [Google Scholar] [CrossRef]
- Yang, C.; Xu, D.; Peng, W.C.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Ti2C3Tx nanosheets as photothermal agents for near-infrared responsive hydrogels. Nanoscale 2018, 10, 15387–15392. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Kornienko, V.; Gogotsi, O.; Oleshko, O.; Kolesnyk, M.; Mishchenko, O.; Zahorodna, V.; Buranich, V.; Pogrebnjak, A.; Zozulia, Y.; et al. Bio-functionalization of Electrospun Polymeric Nanofibers by Ti3CTx MXene. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Application and Properties (NAP), Sumy, Ukraine, 9–13 November 2020; pp. 9–13. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, S.; Cheng, F.; He, X.; Liu, Z.; Wang, W.; Zhou, L.; Zhang, Q. Bioactive anti-inflammatory, antibacterial, conductive multifunctional scaffold based on MXene@CeO2 nanocomposites for infection-impaired skin multimodal therapy. Chem. Eng. J. 2021, 424, 130148. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Liu, Z.; Wang, S.; Liu, Z.; Chen, F.; Zhang, H.; Kong, J.; Zhou, F.; Zhang, Q. Conductive Antibacterial Hemostatic Multifunctional Scaffolds Based on Ti3C2TxMXene Nanosheets for Promoting Multidrug-Resistant Bacteria-Infected Wound Healing. ACS Nano 2021, 15, 2468–2480. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene Nanocomposite Organohydrogel for Flexible, Healable, Low-Temperature Tolerant Strain Sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Park, J.Y. Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition. ACS Appl. Mater. Interfaces 2020, 12, 22212–22224. [Google Scholar] [CrossRef]
- Rafieerad, A.; Sequiera, G.L.; Yan, W.; Kaur, P.; Amiri, A.; Dhingra, S. Sweet-MXene hydrogel with mixed-dimensional components for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 101, 103440. [Google Scholar] [CrossRef]
- Pan, S.; Yin, J.; Yu, L.; Zhang, C.; Zhu, Y.; Gao, Y.; Chen, Y. 2D MXene-Integrated 3D-Printing Scaffolds for Augmented Osteosarcoma Phototherapy and Accelerated Tissue Reconstruction. Adv. Sci. 2020, 7, 1901511. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, G.P.; Maharjan, B.; Shrestha, S.; Bhattarai, D.P.; Yoon, D.; Park, C.H.; Kim, C.S. Synthesis, characterizations, and biocompatibility evaluation of polycaprolactone–MXene electrospun fibers. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124282. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.J.; Li, P.; Zhao, Y.; Niu, Q.J. Fabrication of novel MXene (Ti3C2)/polyacrylamide nanocomposite hydrogels with enhanced mechanical and drug release properties. Soft Matter 2019, 16, 162–169. [Google Scholar] [CrossRef]
- Huang, R.; Chen, X.; Dong, Y.; Zhang, X.; Wei, Y.; Yang, Z.; Li, W.; Guo, Y.; Liu, J.; Yang, Z.; et al. MXene Composite Nanofibers for Cell Culture and Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 2125–2131. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Gao, D.; Wu, C.; Hu, B.; Tan, G.; Du, N.; Cai, X.; Yang, Z.; Zhang, X. NIR-responsive MXene nanobelts for wound healing. NPG Asia Mater. 2021, 13, 24. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A multimodal antimicrobial platform based on MXene for treatment of wound infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, H.; Xu, T.; Zhu, D.; Yin, J.; Chen, Y.; Yu, X.; Gao, J.; Zhang, C.; Chen, Y.; et al. Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small 2020, 16, 1906814. [Google Scholar] [CrossRef]
- Xu, C.; Chang, Y.; Xu, Y.; Wu, P.; Mu, C.; Nie, A.; Qu, Y.; Duan, D.; Guo, X.; Liu, Z.; et al. Silicon-Phosphorus-Nanosheets-Integrated 3D-Printable Hydrogel as a Bioactive and Biodegradable Scaffold for Vascularized Bone Regeneration. Adv. Healthc. Mater. 2021, 11, 2101911. [Google Scholar] [CrossRef]
- Xu, C.; Chang, Y.; Wu, P.; Liu, K.; Dong, X.; Nie, A.; Mu, C.; Liu, Z.; Dai, H.; Luo, Z. Two-Dimensional-Germanium Phosphide-Reinforced Conductive and Biodegradable Hydrogel Scaffolds Enhance Spinal Cord Injury Repair. Adv. Funct. Mater. 2021, 31, 2104440. [Google Scholar] [CrossRef]
- Qian, Y.; Xu, Y.; Yan, Z.; Jin, Y.; Chen, X.; Yuan, W.E.; Fan, C. Boron nitride nanosheets functionalized channel scaffold favors microenvironment rebalance cocktail therapy for piezocatalytic neuronal repair. Nano Energy 2021, 83, 105779. [Google Scholar] [CrossRef]
- Jayakumar, A.; Surendranath, A.; PV, M. 2D materials for next generation healthcare applications. Int. J. Pharm. 2018, 551, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2017, 29, 1603276. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hong, X.; Wang, J.; Feng, L.; Fan, T.; Guo, R.; Zhang, H. 2D Nanomaterials for Tissue Engineering and Regenerative Nanomedicines: Recent Advances and Future Challenges. Adv. Healthc. Mater. 2021, 10, 2001743. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, Z.; Zhao, J.; Wang, F.; Lu, M.; Deng, L.; Cui, W. Black phosphorus-based 2D materials for bone therapy. Bioact. Mater. 2020, 5, 1026–1043. [Google Scholar] [CrossRef]
- Chen, X.; McDonald, A.R. Functionalization of Two-Dimensional Transition-Metal Dichalcogenides. Mater. Views Adv. Mater. 2016, 28, 5738–5746. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Pak, J.; Chung, S.; Lee, T. Recent Advances in Interface Engineering of Transition-Metal Dichalcogenides with Organic Molecules and Polymers. ACS Nano 2019, 13, 9713–9734. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, D.; Liu, F. Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching. Materials 2022, 15, 307. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, N. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef]
- Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as emerging nanofillers for high-performance polymer composites: A review. Compos. Part B 2021, 217, 108867. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Han, J.; Chen, Y.; Lin, H.; Yang, T. Surface Nanopore Engineering of 2D MXenes for Targeted and Synergistic Multitherapies of Hepatocellular Carcinoma. Adv. Mater. 2018, 30, 1706981. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Kaushik, A.; Ranjan, P.; Khan, R.; Srivastava, A.K. Borophene as an emerging 2D flatland for biomedical applications: Current challenges and future prospects. J. Mater. Chem. B 2022, 10, 1146–1175. [Google Scholar] [CrossRef]
- Duo, Y.; Xie, Z.; Wang, L.; Mahmood Abbasi, N.; Yang, T.; Li, Z.; Hu, G.; Zhang, H. Borophene-based biomedical applications: Status and future challenges. Coord. Chem. Rev. 2021, 427, 213549. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Driscoll, N.; Richardson, A.G.; Maleski, K.; Anasori, B.; Adewole, O.; Lelyukh, P.; Escobedo, L.; Cullen, D.K.; Lucas, T.H.; Gogotsi, Y.; et al. Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces. ACS Nano 2018, 12, 10419–10429. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, P.; Yang, S.; Zhou, X.; Wang, Z.; Li, C.; Wang, S.; Zhai, T.; Tao, X. Low-Symmetry and Nontoxic 2D SiP with Strong Polarization-Sensitivity and Fast Photodetection. Adv. Opt. Mater. 2021, 9, 2100198. [Google Scholar] [CrossRef]
- Sar, H.; Gao, J.; Yang, X. 2D layered SiP as anisotropic nonlinear optical material. Sci. Rep. 2021, 11, 6372. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Cuniberti, G.; Zhuang, X. Two-Dimensional SiP, SiAs, GeP and GeAs as Promising Candidates for Photocatalytic Applications. Coatings 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Liu, W.; Zhou, H.; Wei, J.; Mu, C.; Wan, Y.; Yang, X.; Nie, A.; Liu, Z.; Yang, X.; et al. Biodegradable 2D GeP nanosheets with high photothermal conversion efficiency for multimodal cancer theranostics. Chem. Eng. J. 2022, 431, 134176. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, Y.; Gu, Y.; Bowman, L.; Zhao, J.; Ding, M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J. Appl. Toxicol. 2018, 38, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.I.; Costa-Almeida, R.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Carbon nanomaterials for phototherapy of cancer and microbial infections. Carbon N. Y. 2022, 190, 194–244. [Google Scholar] [CrossRef]
- Wu, J.; Yu, Y.; Su, G. Safety Assessment of 2D MXenes: In Vitro and In Vivo. Nanomaterials 2022, 12, 828. [Google Scholar] [CrossRef]
- Fojtů, M.; Teo, W.Z.; Pumera, M. Environmental impact and potential health risks of 2D nanomaterials. Environ. Sci. Nano 2017, 4, 1617–1633. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa-Almeida, R.; Santos, S.G.; Magalhães, F.D.; Pinto, A.M. Advances in carbon nanomaterials for immunotherapy. Appl. Mater. Today 2022, 27, 101397. [Google Scholar] [CrossRef]
- Peng, L.; Abbasi, N.; Xiao, Y.; Xie, Z. Black Phosphorus: Degradation Mechanism, Passivation Method, and Application for In Situ Tissue Regeneration. Adv. Mater. Interfaces 2020, 7, 2001538. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Al-Asmakh, M.; Rasool, K.; Mahmoud, K.A. Ecotoxicological assessment of Ti3C2T:X (MXene) using a zebrafish embryo model. Environ. Sci. Nano 2018, 5, 1002–1011. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Bai, X. Two-Dimensional Transition Metal Dichalcogenides: Synthesis, Biomedical Applications and Biosafety Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and Investigational Nanodrugs. Phys. Ther. 2017, 42, 742–755. [Google Scholar]
- Ruiz, A.; Martín, C.; Reina, G. Nanoscale Advances Does black phosphorus hold potential to overcome graphene oxide ? A comparative review of their promising application for cancer therapy. Nanoscale Adv. R. Soc. Chem. 2021, 3, 4029–4036. [Google Scholar] [CrossRef]
- Alam, S.; Asaduzzaman, M.; Shahid, A.; Alam, R.; Rahim, A. Synthesis of emerging two-dimensional (2D) materials—Advances, challenges and prospects. FlatChem 2021, 30, 100305. [Google Scholar] [CrossRef]
- Yang, L.; Chen, W.; Yu, Q.; Liu, B. Mass production of two-dimensional materials beyond graphene and their applications. Nano Res. 2021, 14, 1583–1597. [Google Scholar] [CrossRef]







| 2DnMat | Composite | Composite Preparation | Application | Outcomes | Ref. |
|---|---|---|---|---|---|
| BP | PVA/pBP Hydrogel | pBP solution added to PVA solution followed by the freezing/thawing method. | NIR-Responsive Drug Release | ↑ mechanical properties ↑ drug loading level (LL) with ↑ pBP% ↑ drug release with NIR exposure No cytotoxic effects observed with 3T3 fibroblasts | [72] |
| PLEL/BP Hydrogel | BP nanosheets were dispersed in a PLEL solution by sonication. | Sprayable Gel for PTT | No cytotoxic effects observed for hMSCs, L929 and HeLa cells. In vivo biocompatibility: no histological abnormalities ↓ tumor recurrence ↓ >99.5% of S. Aureus reduction with NIR irradiation | [73] | |
| Cellulose/BP Hydrogel | Celullose, BP, and epichlorohydrin solutions were mixed. The solution was cross-linked. Dialysis was performed to removed excess reagents. | PTT | ↑ mechanical properties with ↑ BP% No cytotoxic effects observed for B16, SMMC-7721 and J774.1 cells. In vivo biocompatibility: no histological abnormalities or increase of inflammatory cytokines levels ↓ tumor volume | [74] | |
| Agarose/BP@PEG Hydrogel, loaded with DOX | BP@PEG nanosheets, agarose aqueous solution and DOX were mixed and rapidly cooled. | Drug Delivery Induced by PTT | No cytotoxic effects observed for MDA-MB-231, A549, HeLa and B16 cells ↑ drug release with NIR exposure ↓ MDA-MB-231 cells with NIR induced drug release In vivo biocompatibility: no histological abnormalities ↓ tumor volume with combination of drug delivery + PTT | [75] | |
| BP/PEA/GelMA Hydrogel | A GelMA and PEA solution was submitted to photopolymerization. BP nanosheets were added to the hydrogel, followed by UV irradiation. | Bone Regeneration | ↑ water-absorption capacity No cytotoxic effects were observed for hDPSCs cells ↑ mineralization and ↑ osteogenic differentiation of hDPSCs In vivo: Newly formed vessels were detectable in 4 weeks and after 12 weeks, the bone defect was completely repaired | [76] | |
| Fibrin/BP Gel | Fibrinogen solutions with BP nanosheets were mixed with thrombin through spraying. | Diabetic Ulcer Treatment + Analgesic + Antibacterial PTT | ↑ gelation time with ↑ BP% ↑ proliferation and differentiation HUVECs ↓ ~94.3% of bacteria with NIR ↑ drug release triggered by NIR ↑ wound healing in vivo with the combination of composite + drug delivery + NIR ↓ 50% wound area in 5.7 days | [77] | |
| BG/BP 3D-printed Scaffold | BG scaffolds were 3D-printed and subsequently soaked in a BP absolute ethyl alcohol solution. | PTT + Bone Regeneration | ↑ proliferation of Saos-2 cells ↓ tumor recurrence ↑ osteogenic differentiation of hBMSCs ↑ in vivo bone tissue formation. | [78] | |
| GelMA/BP@PDA Hydrogel | BP@PDA and GelMA solutions were sonicated until homogeneous, followed by UV irradiation. | MSCs Differentiation | ↑ BP@PDA ↓ the swelling ratio of the hydrogel ↓ impedance for ↑ BP% ↓ degradation rate, presumably due to the functionalization with PDA that stabilizes the network Electrical stimulation ↑ MSCs proliferation ↑ neuronal gene expression in vitro Contrary to in vitro assays, the degradation of the composite is faster in vivo. | [79] | |
| BP/PCL Nanoscaffold | BP nanoplates were incorporated in a PCL dicloromethane solution. The solution was sprayed onto a conduit shaped mold. | Neural Regeneration | ↑ electrical conductivity with ↑ %BP In vivo biocompatibility: no histological abnormalities and no increase of apoptotic cell markers No increase of blood biochemical parameters 6 months post-implatation ↑ angiogenesis | [80] | |
| MoS2 | PLGA/MoS2@PEG/DOX Injectable Implant | PLGA was dispersed in NMP, MoS2 was dispersed in the PLGA/NMP solution and DOX was dissolved in the PLGA/MoS2 dispersion. | Drug Delivery + PTT | No cytotoxic effects were observed for L929 cells. No increased blood coagulation 95% of DOX drug was loaded onto PLGA ↑ drug release with NIR exposure ↓ tumor volume actively reduced with NIR | [81] |
| PAN/MoS2 Nanofibers | PAN was added to a MoS2/N,N-dimethylformamide solution and electrospun. | Composite Effects on BMSCs | ↑ %MoS2 ↑ nanofiber surface roughness The fibers presented very low cytotoxicity even at 40% MoS2 and high rates of cell attachment 40% MoS2 ↑ osteogenic differentiation | [82] | |
| Ti3C2Tx | PLA/Ti3C2Tx@OTES Membrane | Solvent casting was used to embed Ti3C2Tx@OTES in PLA. | Bone Regeneration | ↑ mechanical properties of the membrane are increased prior to saturation of the filler ↑ MC3T3-E1 cell adhesion and ↑ proliferation ↑ Osteogenic differentiation | [83] |
| PNIPAM/Ti3C2Tx Hydrogel | PNIPAM and a cross-linker were added to a Ti3C2Tx solution and purged, followed by the addition of a polymerization accelerator. | PTT | MXene did not disrupt the hydrogel network Good photothermal stability Promising prospects for biomedical and drug delivery | [84] | |
| PLA/Ti3C2Tx Nanofibers | Electrospun PLA was immersed in a Ti3C2Tx solution. | Antibacterial | ↑ roughness of the nanofibers No cytotoxic effects were observed with U2OS cells ↓ adhesion of S. aureus bacteria | [85] | |
| Chitosan/Ti3C2Tx Nanofibers | Ti3C2Tx was loaded onto a chitosan solution and electrospun. | Antibacterial Wound Dressing | Stable electrospinning GA crosslinked composite fibers exhibited a bacterial reduction of 95% for E. Coli and 62% for S. aureus No cytotoxic effects were observed with HeLa cells | [68] | |
| F127-PEI-OSA/Ti3C2Tx@CeO2 Hydrogel | A solution of F127-PEI, OSA, and Ti3C2Tx@CeO2 was prepared was kept at 37 °C. | Multifunctional Wound Healing Scaffold | No cytotoxic effects were observed with L929 cells ↓ ROS with the addition of Ti3C2Tx@CeO2 ↑ fibroblast proliferation with composite + ES ↑ healing on in vitro scratch assay: within 24 h, composite + ES reduced the unhealed portion by 73.6% ↓ 100% of bacterial colonies ↑ wound healing and antibacterial in vivo ↑ anti-inflammatory cytokines In vivo biocompatibility: no histological abnormalities | [86] | |
| PGE/HCHO/Ti3C2Tx@PDA Scaffold | Ti3C2Tx@PDA and PEG solutions were mixed in a HCHO solution and vortexed until homogenous. | Multifunctional Antibacterial Wound Healing | L929 cells adhered to the scaffolds ↓ bacteria growth of 98.6% for E. coli, 99.9% for S. aureus and 99.03% for MRSA ↓ coagulation time in vivo In vivo biocompatibility: no histological abnormalities ↑ wound healing observed by ↑ α-actin, COL III, and VEGF | [87] | |
| PAAm-PVA/Ti3C2Tx Hydrogel | The individual components that comprise the hydrogel were mixed in a aqueous solution with an initiator, followed by the addition of borax until a gel is formed. | Biosensor | ↑ hydrogel conductivity ↑ antifreezing properties ↑ sensitivity to monitor human activities | [88] | |
| PVA/Ti3C2Tx Hydrogel | A Ti3C2Tx solution was mixed with a PVA solution, followed by the addition of borax. | Biosensor, Electronic skin | ↑ hydrogel stretchability self-healing ability remains at 97.4% after 5 cycles of cutting ↑ sensitivity, it detects swallowing and finger motion | [89] | |
| PVDF-TrFE/Ti3C2Tx Nanofibers | Ti3C2Tx was added to a PVDF-TrFE solution and electrospun. | Biosensor for Physiological Signal Acquisition | The sensor showed good capabilities in recognizing pulse signals in the wrist, breathing, and promising results for future aiding of Parkinson’s diagnose by measuring unnoticeable resting tremor in hands | [90] | |
| Ti3C2 | Ti3C2/Honey/Chitosan Hydrogel | Ti3C2 was added to a chitosan hydrogel solution, followed by the addition of honey, β-glycerophosphate and hydroxyethyl cellulose. | Biomedical Applications | Good swelling ability, biodegradable, and self-healing No cytotoxic effects were observed for MSCs and iPSCs cells | [91] |
| BG/Ti3C2 3D-Printed Scaffolds | 3D-printed scaffolds were soaked in Ti3C2 aqueous solution. | PTT + Bone Regeneration | No cytotoxic effects were observed for Saos-2 cells ↑ adhesion and ↑ proliferation of hBMSCs ↑ osteogenic capability In vivo biocompatibility: no histological abnormalities ↑ differentiation from ↑ of COL I, RUNX2, OCN and OPN gene expression ↑ bone regeneration; ↑ tissue calcification | [92] | |
| PCL/Ti3C2 Electrospun Scaffolds | PCL was added to Ti3C2 was dispersed on a dimethylformamide and chloroform solution and electrospun. | Biomedical Applications—study | ↑ fiber diameter with ↑ MXene content ↑ biomineralization in vitro The scaffold is more biocompatible for MC3T3-E1 than NIH-3T3 cells Promising prospects for wound healing, bone TE and cancer therapy | [93] | |
| Cellulose/Ti3C2 Hydrogel | The same method as described in [74]. | PTT + Drug Release | No cytotoxic effects were observed for HepAl-6, SMMC-7721, HepG2, U-118MG and U-251MG cells. NIR irradiation yielded in ~100% killing efficiency of tumor cells In vivo biocompatibility: no histological abnormalities Dual modal PTT/chemo was successful in vivo as it completely eliminated tumor cells | [94] | |
| PAM/Ti3C2 Hydrogel | The hydrogel was prepared using a free radical polymerization method. An aqueous Ti3C2 solution was mixed with acrylamide and an initiator to initiate polymerization. | Drug Release | ↑ mechanical properties of the hydrogel ↑ drug loads and ↑ drug release | [95] | |
| PLLA-PHA/Ti3C2 Nanofibers | PLLA and PHA were added to a Ti3C2 dichloromethane/dimethylformamide solution. | Tissue Engineering | ↑ adhesion and slightly ↑ proliferation of BMSCs Cells could grow on both scaffolds (w/ and w/o MXene) but on the pristine nanofibers the cells presented a contraction state. The composite nanofibers present, overall, a positive role on BMSCs growth and can enhance osteogenic ability | [96] | |
| PAN-PVP/Ti3C2@PAAV Fibrous Nanobelts | PAN and PVP were added to a Ti3C2 dimethylformamide solution and electrospun. The nanofibers were soaked in a PAAV aqueous solution. | Wound Healing + Drug delivery | ↑ adhesion and ↑ proliferation of BMSCs ↑ vitamin E release with NIR radiation ↑ wound healing with NIR in vivo, displaying the advantage of vitamin E release | [97] | |
| PVA/AMX/Ti3C2 Nanofibrous Membrane | PVA was dissolved in a Ti3C2 aqueous solution, followed by the addition of AMX. The final solution was electrospun. | Wound Healing + Drug Delivery | No cytotoxic effects were observed for L929 cells ↑ AMX release with NIR radiation ↑ antibacterial properties with NIR: ↓ 96.1% for E. coli and ↓ 99.1% for S. aureus In vivo biocompatibility: no histological abnormalities with NIR↑ wound healing rates and neglectable inflammation | [98] | |
| Nb2C | BG/Nb2C@Silica 3D-printed Scaffold | A mesoporous silica layer was coated onto Nb2C nanosheets. 3D-printed BG scaffolds were soaked on a Nb2C@Silica solution. | Bone Regeneration | ↑ NO release is with NIR radiation, maintaining a slow-release profile after No cytotoxic effects were observed for Saos-2 cells. In vivo tests were carried out in mice. NO release + photothermal therapy yielded the better results. ↑ adhesion and ↑ proliferation for hBMSCs ↑ osteoindcution properties in vivo ↑ increased calcified tissue | [99] |
| SiP | GelMA-PEGDA/SiP@AC 3D-printed Hydrogel | GelMA, PEGDA, and SiP@AC were mixed in a phosphate-buffered saline solution and 3D-printed. | Bone Regeneration | ↑ release of P ions No cytotoxic effects below 0.5% SiP@AC ↑ ALP expression ↑ mineralized nodules ↑ osteogenic differentiation markers (Opn, Runx2 and Col-I) ↑ angiogenic genes (VEGF and bFGF) ↑ in vivo bone growth In vivo biocompatibility: no histological abnormalities | [100] |
| GeP | HA-DA/GeP@PDA Injectable Hydrogel | HA-DA and GeP@PDA aqueous solutions were mixed. Horseradish peroxidase was added as an initiator for the cross-link of the hydrogel. | Spinal Cord Injury Repair | ↓ swelling ratio but ↑ conductivity No cytotoxic effects were observed for concentrations below 0.5% of GeP@PDA ↑ NSCs differentiation ↑ coordination movements in vivo ↓ spinal cord cavity ↑ anti-inflammatory factor IL-10 and ↓ TNF-α 6 weeks post-surgery ↑ levels of CD31-labeled vascular endothelial cells ↓ invasion of lesion area and ↑ secretion of vascular endothelial growth factor ↑ angiogenesis enhanced by P element released from the GeP nanosheets. P ↑ Akt protein, MMP-2 and bFGF expression, which enhances new blood vessel formation | [101] |
| Boron Nitride (BN) | BN/PCL Scaffold | BN nanosheets were added to a PLC/dichloromethane solution and sprayed onto a rotatory mould. | Nerve Regeneration | ↑ mechanical properties with ↑ BN% No cytotoxic effects were observed for RSC96 cells ↓ immune response In vivo biocompatibility: no histological abnormalities ↑ S100 and Tuj1 | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, L.S.; Magalhães, F.D.; Pinto, A.M. New Polymeric Composites Based on Two-Dimensional Nanomaterials for Biomedical Applications. Polymers 2022, 14, 1464. https://doi.org/10.3390/polym14071464
Pires LS, Magalhães FD, Pinto AM. New Polymeric Composites Based on Two-Dimensional Nanomaterials for Biomedical Applications. Polymers. 2022; 14(7):1464. https://doi.org/10.3390/polym14071464
Chicago/Turabian StylePires, Laura S., Fernão D. Magalhães, and Artur M. Pinto. 2022. "New Polymeric Composites Based on Two-Dimensional Nanomaterials for Biomedical Applications" Polymers 14, no. 7: 1464. https://doi.org/10.3390/polym14071464
APA StylePires, L. S., Magalhães, F. D., & Pinto, A. M. (2022). New Polymeric Composites Based on Two-Dimensional Nanomaterials for Biomedical Applications. Polymers, 14(7), 1464. https://doi.org/10.3390/polym14071464