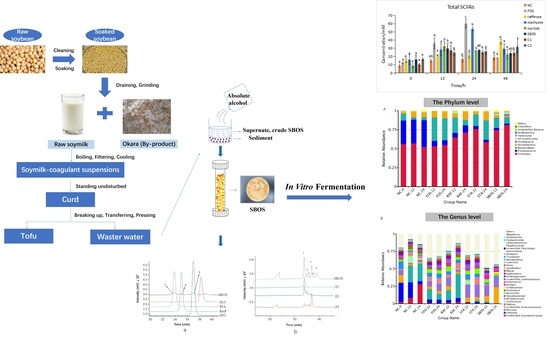
In Vitro Fermentability of Soybean Oligosaccharides from Wastewater of Tofu Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Isolation and Fractionation of SBOS
2.3. Oligosaccharide Profile Analysis
2.4. In Vitro Fermentation of SBOS
2.4.1. Preparation of Human Intestinal Microbiota and Medium
2.4.2. In Vitro Fermentation
2.5. Determination of SCFAs
2.6. Changes in Mw of SBOS
2.7. 16S rDNA Gene Sequencing of Gut Microbiota
2.8. Statistical Analysis
3. Results
3.1. Isolation and Fractionation
3.2. In Vitro Fermentation
3.2.1. SCFAs Production
3.2.2. Changes in Mw during Fermentation
3.2.3. Changes in Microbiota Composition
3.3. Correlation of Structure and Prebiotic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, J.-Y.; Liu, S.-Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Fung, W.-Y.; Liong, M.-T. Evaluation of proteolytic and ACE-inhibitory activity of Lactobacillus acidophilus in soy whey growth medium via response surface methodology. LWT 2010, 43, 563–567. [Google Scholar] [CrossRef]
- Yang, Y.; Awasthi, M.K.; Ren, X.; Guo, H.; Lv, J. Effect of bean dregs on nitrogen transformation and bacterial dynamics during pig manure composting. Bioresour. Technol. 2019, 288, 121430. [Google Scholar] [CrossRef] [PubMed]
- Matemu, A.O.; Kayahara, H.; Murasawa, H.; Nakamura, S. Importance of size and charge of carbohydrate chains in the preparation of functional glycoproteins with excellent emulsifying properties from tofu whey. Food Chem. 2009, 114, 1328–1334. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, M.; Wang, K.; Zhang, Z.; Shah, N.P.; Wei, H. Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors—A review. Carbohydr. Polym. 2022, 284, 119043. [Google Scholar] [CrossRef]
- Zhou, X.L.; Kong, X.F.; Yang, X.J.; Yin, Y.L. Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J. Anim. Sci. 2012, 90, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Fei, B.-B.; Ling, L.; Hua, C.; Ren, S.-Y. Effects of soybean oligosaccharides on antioxidant enzyme activities and insulin resistance in pregnant women with gestational diabetes mellitus. Food Chem. 2014, 158, 429–432. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, S.; Ma, J. Evaluation of cardio-protective effect of soybean oligosaccharides. Gene 2015, 555, 329–334. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, X.; Yang, J.; Giovanni, V.; Wang, C. Impacts of functional oligosaccharide on intestinal immune modulation in immunosuppressive mice. Saudi J. Biol. Sci. 2020, 27, 233–241. [Google Scholar] [CrossRef]
- Nakata, T.; Kyoui, D.; Takahashi, H.; Kimura, B.; Kuda, T. Inhibitory effects of soybean oligosaccharides and water-soluble soybean fibre on formation of putrefactive compounds from soy protein by gut microbiota. Int. J. Biol. Macromol. 2017, 97, 173–180. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Yang, C.; Wang, Z. Enzymatic preparation and characterization of soybean oligosaccharides from Okara. Procedia Eng. 2012, 37, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.Y.; Li, X.; Zhu, X.; Dong, W.G.; Yang, G.Q. Soybean oligosaccharides attenuate odour compounds in excreta by modulating the caecal microbiota in broilers. Animal 2021, 15, 100159. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, L.-J.; Zhu, J.-J.; Bo, X.; Rui, L. Chemical composition analysis of soybean oligosaccharides and its effect on ATPase activities in hyperlipidemic rats. Int. J. Biol. Macromol. 2010, 46, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Iwasaki, K.-I.; Nakajima, M.; Nabetani, H.; Nakao, S.-I. Recovery of oligosaccharides from steamed soybean waste water in tofu processing by reverse osmosis and nanofiltration membranes. Biosci. Biotechnol. Biochem. 1996, 60, 421–428. [Google Scholar] [CrossRef]
- Wang, N.; Jia, G.; Wang, C.; Chen, M.; Xie, F.; Nepovinnykh, N.V.; Goff, H.D.; Guo, Q. Structural characterization and immunomodulatory activity of exopolysaccharides from liquid fermentation of Monascus purpureus (Hong Qu). Food Hydrocoll. 2020, 103, 105636. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Nam, Y.; Jung, M.; Roh, S.W.; Kim, M.; Bae, J.; Dias-Neto, E. Comparative analysis of Korean human gut microbiota by barcoded pyrosequencing. PLoS ONE 2011, 6, e22109. [Google Scholar] [CrossRef]
- Ding, H.H.; Cui, S.W.; Goff, H.D.; Gong, J. Short-chain fatty acid profiles from flaxseed dietary fibres after in vitro fermentation of pig colonic digesta: Structure–function relationship. Bioact. Carbohydr. Diet. Fibre 2015, 6, 62–68. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, S. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Polym. 2000, 41, 133–141. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Shen, N.; Zhang, F.; Zeng, R.J. High-purity propionate production from glycerol in mixed culture fermentation. Bioresour. Technol. 2016, 219, 659–667. [Google Scholar] [CrossRef]
- Lan, Y.; Williams, B.A.; Verstegen, M.W.A.; Patterson, R.; Tamminga, S. Soy oligosaccharides in vitro fermentation characteristics and its effect on caecal microorganisms of young broiler chickens. Anim. Feed Sci. Technol. 2007, 133, 286–297. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Wang, Q.; Xu, Q.; Zhang, Y.; Lin, B.; Guan, X.; Qian, L.; Zheng, Y. In vitro fermentation of O-acetyl-arabinoxylan from bamboo shavings by human colonic microbiota. Int. J. Biol. Macromol. 2019, 125, 27–34. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Arseneau, K.O.; Tamagawa, H.; Pizarro, T.T.; Cominelli, F. Innate and adaptive immune responses related to IBD pathogenesis. Curr. Gastroenterol. Rep. 2007, 9, 508–512. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- He, L.; Zhang, F.; Jian, Z.; Sun, J.; Chen, J.; Liapao, V.; He, Q. Stachyose modulates gut microbiota and alleviates dextran sulfate sodium-induced acute colitis in mice. Saudi J. Gastroenterol. 2020, 26, 153. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Catabolism of Saccharina japonica polysaccharides and oligosaccharides by human fecal microbiota. LWT 2020, 130, 109635. [Google Scholar] [CrossRef]
- Martin, J.K.; Jinmo, K.; Francis, F.B.; Daniel, D.G.; Linda, J.B. Carbohydrate source and bifidobacteria influence the growth of Clostridium perfringens in vivo and in vitro. Nutr. Res. 1998, 18, 1889–1897. [Google Scholar]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J. Agr. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef]
- Voragen, A.G.J. Technological aspects of functional food-related carbohydrates. Trends Food Sci. Techol. 1998, 9, 328–335. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, C.; Zhang, L.; Wang, Y.; Chen, G.; Fan, J.; Jia, Y.; Yan, F.; Ning, C. Pectin oligosaccharides from fruit of Actinidia arguta: Structure-activity relationship of prebiotic and antiglycation potentials. Carbohydr. Polym. 2019, 217, 90–97. [Google Scholar] [CrossRef]
- Mueller, M.; Čavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Lu, Y.; Hao, H.; Liu, J.; Huang, R. Structural features, interaction with the gut microbiota and anti-tumor activity of oligosaccharides. RSC Adv. 2020, 10, 16339–16348. [Google Scholar] [CrossRef]








| Fractions | Yield (%) | Peak Area Ratios (%) | ||
|---|---|---|---|---|
| Stachyose 1 | Raffinose 2 | Sucrose 3 | ||
| Z1 | 14 | 100.00 | - | - |
| Z2 | 13 | 60.41 | 28.07 | 11.52 |
| Z3 | 17 | 19.25 | 13.06 | 67.69 |
| SCFAs | Standard Curve | R2 |
|---|---|---|
| Acetic acid | y = 0.1556x − 0.0057 | 0.9916 |
| Propionic acid | y = 0.3400x − 0.0216 | 0.9905 |
| Isobutyric acid | y = 0.4474x − 0.0257 | 0.9947 |
| Butyric acid | y = 0.4396x − 0.0282 | 0.9924 |
| Isovaleric acid | y = 0.4801x − 0.0259 | 0.9958 |
| Valeric acid | y = 0.4696x − 0.0413 | 0.9921 |
| Isocaproic acid | y = 0.4630x − 0.0359 | 0.9959 |
| Caproic acid | y = 0.4582x − 0.0359 | 0.9953 |
| Heptanoic acid | y = 0.4388x − 0.0310 | 0.9950 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, C.; Shan, Z.; Yin, S.; Wang, Y.; Wang, C.; Liu, T.; Wang, N.; Guo, Q. In Vitro Fermentability of Soybean Oligosaccharides from Wastewater of Tofu Production. Polymers 2022, 14, 1704. https://doi.org/10.3390/polym14091704
Wang Y, Li C, Shan Z, Yin S, Wang Y, Wang C, Liu T, Wang N, Guo Q. In Vitro Fermentability of Soybean Oligosaccharides from Wastewater of Tofu Production. Polymers. 2022; 14(9):1704. https://doi.org/10.3390/polym14091704
Chicago/Turabian StyleWang, Yuling, Chunrong Li, Zhengxin Shan, Sijia Yin, Yue Wang, Congcong Wang, Tianhui Liu, Nifei Wang, and Qingbin Guo. 2022. "In Vitro Fermentability of Soybean Oligosaccharides from Wastewater of Tofu Production" Polymers 14, no. 9: 1704. https://doi.org/10.3390/polym14091704
APA StyleWang, Y., Li, C., Shan, Z., Yin, S., Wang, Y., Wang, C., Liu, T., Wang, N., & Guo, Q. (2022). In Vitro Fermentability of Soybean Oligosaccharides from Wastewater of Tofu Production. Polymers, 14(9), 1704. https://doi.org/10.3390/polym14091704