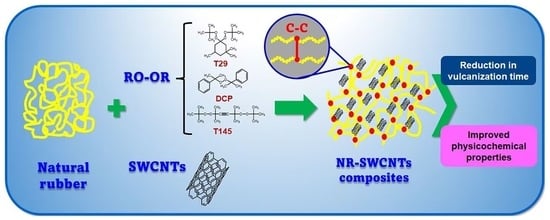
Effect of Single-Walled Carbon Nanotubes on the Cross-Linking Process in Natural Rubber Vulcanization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dispersion of SWCNTs in NR
2.3. Preparation of NR Composites with Sulfur
2.4. Preparation of NR Composites with Organic Peroxides
2.5. Vulcanization–Compression Molding Process
2.6. Determination of Temperature and Time (t.v.) of Vulcanization of NR Composites
2.7. Characterization Techniques
2.7.1. Electron Microscopy
2.7.2. Mechanical Properties
2.7.3. Raman Spectroscopy
2.8. Determination of Average Molecular Weight between Cross-Link Points ()
3. Results and Discussion
3.1. Analysis of NR Vulcanization Process
3.2. Microstructural Analysis
3.3. Mechanical Properties of NR Composites Using Different Cross-Linking Agents
3.3.1. Tensile Properties
3.3.2. Tear Strength and Hardness
3.4. Evaluation of the Degree of Vulcanization of NR Composites
3.5. Raman Spectroscopy of NR-SWCNTs Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, S.; Thomas, S.; Abraham, J.; George, S.C.; Thomas, S. Investigation of the mechanical, thermal and transport properties of NR/NBR blends: Impact of organoclay content. J. Polym. Res. 2018, 25, 165. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part I: Effect of temperature and peroxide concentration. J. Polym. Eng. 2014, 34, 617–624. [Google Scholar] [CrossRef]
- Valentín, J.L.; Rodríguez, A.; Marcos-Fernández, A.; González, L. Dicumyl peroxide cross-linking of nitrile rubbers with different content in acrylonitrile. J. Appl. Polym. Sci. 2005, 96, 1–5. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulfur and peroxide curing of rubber compounds based on NR and NBR. Part I: Cross-linking and physical-mechanical properties. Elastom. Und Kunstst.-Elastomers Plast. 2017, 1–2, 27–33. [Google Scholar]
- Naebpetch, W.; Junhasavasdikul, B.; Saetung, A.; Tulyapitak, T.; Nithi-Uthai, N. Influence of accelerator/sulphur and co-agent/peroxide ratios in mixed vulcanisation systems on cure characteristics, mechanical properties and heat aging resistance of vulcanised SBR. Plast. Rubber Compos. 2016, 45, 436–444. [Google Scholar] [CrossRef]
- Mathew, G.; Singh, R.P.; Nair, N.R.; Thomas, S. Recycling of natural rubber latex waste and its interaction in epoxidized natural rubber. Polymer 2000, 42, 2137–2165. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Hudec, I. Peroxide curing systems applied for crosslinking of rubber compounds based on SBR. Adv. Ind. Eng. Polym. Res. 2020, 3, 120–128. [Google Scholar] [CrossRef]
- Kurian, T.; De, P.P.; Khastgir, D.; Tripathy, D.K.; De, S.K.; Peiffer, D.G. Reinforcement of EPDM-based ionic thermoplastic elastomer by carbon black. Polymer 1995, 36, 3875–3884. [Google Scholar] [CrossRef]
- Fei, Z.; Long, C.; Qingyan, P.; Shugao, Z. Influence of carbon black on crosslink density of natural rubber. J. Macromol. Sci. Phys. 2012, 51, 1208–1217. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of carbon black reinforcement of rubber: Perspective on the original polymer nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef]
- Yatsuyanagi, F.; Suzuki, N.; Ito, M.; Kaidou, H. Effects of secondary structure of fillers on the mechanical properties of silica filled rubber systems. Polymer 2001, 42, 9523–9529. [Google Scholar] [CrossRef]
- Alberola, N.D.; Benzarti, K.; Bas, C.; Bomal, Y. Interface effects in elastomers reinforced by modified precipitated silica. Polym. Compos. 2001, 22, 312–325. [Google Scholar] [CrossRef]
- Nematollahi, M.; Jalali-Arani, A.; Golzar, K. Organoclay maleated natural rubber nanocomposite. Prediction of abrasion and mechanical properties by artificial neural network and adaptive neuro-fuzzy inference. Appl. Clay. Sci. 2014, 97–98, 187–199. [Google Scholar] [CrossRef]
- López-Manchado, M.A.; Arroyo, M.; Herrero, B.; Biagiotti, J. Vulcanization kinetics of natural rubber-organoclay nanocomposites. J. Appl. Polym. Sci. 2003, 89, 1–15. [Google Scholar] [CrossRef]
- Kim, J.S.; Reneker, D.H. Mechanical properties of composites using ultrafine electrospun fibers. Polym. Compos. 1999, 20, 124–131. [Google Scholar] [CrossRef]
- Cooper, C.A.; Ravich, D.; Lips, D.; Mayer, J.; Wagner, H.D. Distribution and alignment of carbon nanotubes and nanofibrils in a polymer matrix. Compos. Sci. Technol. 2002, 62, 1105–1112. [Google Scholar] [CrossRef]
- López-Manchado, M.A.; Biagiotti, J.; Valentini, L.; Kenny, J.M. Dynamic mechanical and Raman spectroscopy studies on interaction between single-walled carbon nanotubes and natural rubber. J. Appl. Polym. Sci. 2004, 92, 3394–3400. [Google Scholar] [CrossRef]
- Zhao, Q.; Tannenbaum, R.; Jacob, K.I. Carbon nanotubes as Raman sensors of vulcanization in natural rubber. Carbon 2006, 44, 1740–1745. [Google Scholar] [CrossRef]
- Anand, K.A.; Jose, T.S.; Alex, R.; Joseph, R. Natural rubber-carbon nanotube composites through latex compounding. Int J Polym. Mater. 2009, 59, 33–44. [Google Scholar] [CrossRef]
- Nah, C.; Lim, J.Y.; Cho, B.H.; Hong, C.K.; Gent, A.N. Reinforcing rubber with carbon nanotubes. J. Appl. Polym. Sci. 2010, 118, 1574–1581. [Google Scholar] [CrossRef]
- Bokobza, L. Enhanced electrical and mechanical properties of multiwall carbon nanotube rubber composites. Polym. Adv. Technol. 2012, 23, 1543–1549. [Google Scholar] [CrossRef]
- Sagar, S.; Iqbal, N.; Maqsood, A.; Bassyouni, M. MWCNTS incorporated natural rubber composites: Thermal insulation, phase transition and mechanical properties. Int. J. Eng. Technol. 2014, 6, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Elango, N.; Gupta, N.S.; Jiun, Y.L.; Golshahr, A. The effect of high loaded multiwall carbon nanotubes in natural rubber and their nonlinear material constants. J. Nanomater. 2017, 2017, 6193961. [Google Scholar] [CrossRef] [Green Version]
- Medupin, R.O.; Abubakre, O.K.; Abdulkareem, A.S.; Muriana, R.A.; Abdulrahman, A.S. Carbon nanotube reinforced natural rubber nanocomposite for anthropomorphic prosthetic foot purpose. Sci. Rep. 2019, 9, 20146. [Google Scholar] [CrossRef] [Green Version]
- Capezza, A.; Andersson, R.L.; Ström, V.; Wu, Q.; Sacchi, B.; Farris, S.; Hedenqvist, M.S.; Olsson, R.T. Preparation and comparison of reduced graphene oxide and carbon nanotubes as fillers in conductive natural rubber for flexible electronics. ACS Omega 2019, 4, 3458–3468. [Google Scholar] [CrossRef]
- Krainoi, A.; Kummerloewe, C.; Vennemann, N.; Nakaramontri, Y.; Pichaiyut, S.; Nakason, C. Effect of carbon nanotubes decorated with silver nanoparticles as hybrid filler on properties of natural rubber nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47281. [Google Scholar] [CrossRef]
- García, D.B.; Mansilla, M.A.; Crisnejo, M.; Farabollini, H.; Escobar, M.M. Effect of carbon nanotubes content on the vulcanization kinetic in styrene-butadiene rubber compounds. Polym. Eng. Sci. 2019, 59, E327–E336. [Google Scholar] [CrossRef]
- Cantournet, S.; Boyce, M.C.; Tsouc, A.H. Micromechanics and macromechanics of carbon nanotube-enhanced elastomers. J. Mech. Phys. Solids 2007, 55, 1321–1339. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Tao, X.; Liu, Y. Carbon nanotube-reinforced polyurethane composite fibers. Compos. Sci. Technol. 2006, 66, 3029–3034. [Google Scholar] [CrossRef]
- Dixon, K.W. Decomposition rates of organic free radical initiators. In Polymer Handbook; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1999; p. II-1, II–28, II-29, II-47. [Google Scholar]
- hFlory, P.J.; Rehner, J. Statistical mechanics of cross- linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Martinez, A.; Francisco-Marquez, M.; Galano, A. Effect of different functional groups on the free radical scavenging capability of single-walled carbon nanotubes. J. Phys. Chem. C 2010, 114, 14734–14739. [Google Scholar] [CrossRef]
- Khizhnyak, P.E.; Fedoseev, S.D.; Lutkov, A.I. Contact thermal resistance of some graphites. J. Eng. Phys. 1977, 33, 1055–1058. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. Phys. 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Choi, S.S.; Han, D.H.; Ko, S.W.; Lee, H.S. Thermal aging behaviors of elemental sulfur-free polyisoprene vulcanizates. Bull. Korean Chem. Soc. 2005, 26, 1853–1855. [Google Scholar]
- Dondi, D.; Buttafava, A.; Zeffiro, A.; Palamini, C.; Lostritto, A.; Giannini, L.; Faucitano, A. The mechanisms of the sulphur-only and catalytic vulcanization of polybutadiene: An EPR and DFT study. Eur. Polym. J. 2015, 62, 222–235. [Google Scholar] [CrossRef]
- Fu, X.; Huang, G.; Xie, Z.; Xing, W. New insights into reinforcement mechanism of nanoclay-filled isoprene rubber during uniaxial deformation by in situ synchrotron X-ray diffraction. RSC Adv. 2015, 5, 25171–25182. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds-overview. Chem. Pap. 2016, 70, 1533. [Google Scholar] [CrossRef]
- Przybysz, M.; Marć, M.; Klein, M.; Saeb, M.R.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Dicumyl Peroxide used as curing agent for different types of rubber matrices Part I: Effect of temperature. Rohst. Und Anwend.-Raw Mater. Appl. 2020, 10, 36–42. [Google Scholar]
- Saito, R.; Grüneis, A.; Samsonidze, G.G.; Brar, V.W.; Dresselhaus, G.; Dresselhaus, M.S.; Jorio, A.; Cançado, L.G.; Fantini, C.; Pimenta, M.A. Double resonance Raman spectroscopy of single-wall carbon nanotubes. New J. Phys. 2003, 5, 157. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Rao, R.; Reppert, J.; Podila, R.; Zhang, X.; Rao, A.M.; Talapatra, S.; Maruyama, B. Double resonance Raman study of disorder in CVD-grown single-walled carbon nanotubes. Carbon 2011, 49, 1318–1325. [Google Scholar] [CrossRef]
- Barros, E.B.; Jorio, A.; Samsonidze, G.G.; Capaz, R.B.; Souza Filho, A.G.; Mendes Filho, J.; Dresselhaus, G.; Dresselhaus, M.S. Review on the symmetry-related properties of carbon nanotubes. Phys. Rep. 2006, 431, 261–302. [Google Scholar] [CrossRef]
- Corio, P.; Santos, P.S.; Brar, V.W.; Samsonidze, G.G.; Dresselhaus, M.S. Potential dependent surface Raman spectroscopy of single wall carbon nanotube films on platinum electrodes. Phys. Lett. 2003, 370, 675–682. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]









| Property | T29 | DCP | T145 |
|---|---|---|---|
| Melting point (°C) | −20 | 39–41 | 88 |
| Boiling point (°C at 1 atm) | 403.47 | 130 | 348.77 |
| Density (g·cm−3) | 0.895 | 1.56 | 1.26 |
| Component | NR (phr) | CB (phr) | SWCNTs (phr) |
|---|---|---|---|
| NR | 100 | 100 | 100 |
| Zinc oxide | 5 | 5 | 5 |
| Stearic Acid | 1 | 1 | 1 |
| Sulfur | 2.5 | 2.5 | 2.5 |
| MBTS | 1 | 1 | 1 |
| PBN | 1 | 1 | 1 |
| CB | - | 0.7 | - |
| SWCNTs | - | - | 0.7 |
| Component | NR (phr) | CB (phr) | SWCNTs (phr) |
|---|---|---|---|
| NR | 100 | 100 | 100 |
| Organic peroxide 1 | 2.5 | 2.5 | 2.5 |
| CB | - | 0.7 | - |
| SWCNTs | - | - | 0.7 |
| Sample | t.v. (s) (% Time Reduction) | |||
|---|---|---|---|---|
| Sulfur (160 °C) | T29 (129 °C) | DCP (160 °C) | T145 (173 °C) | |
| Calculated 1 | - | 382 | 255 | 368 |
| Neat | 600 | 380 | 252 | 368 |
| CB | 480 (20) | 312 (18.3) | 240 (5.9) | 360 (2.2) |
| SWCNTs | 300 (50) | 261 (31.7) | 198 (22.4) | 306 (16.8) |
| Sample ID | Tensile Strength (MPa) | Strain at Breakage (%) | Modulus (MPa) | |
|---|---|---|---|---|
| 100% | 300% | |||
| NR-S | 14.77 | 800 | 0.87 | 2.50 |
| NR-S-CB | 10.39 | 600 | 0.95 | 2.84 |
| NR-S-SWCNTs | 25.23 | 790 | 1.10 | 4.05 |
| NR-T29 | 4.52 | 651 | 0.36 | 1.14 |
| NR-T29-CB | 2.82 | 687 | 0.34 | 1.07 |
| NR-T29-SWCNTs | 7.90 | 564 | 0.66 | 1.61 |
| NR-DCP | 7.25 | 600 | 0.86 | 2.56 |
| NR-DCP-CB | 8.77 | 516 | 0.83 | 2.78 |
| NR-DCP-SWCNTs | 10.14 | 495 | 0.88 | 2.98 |
| NR-T145 | 5.52 | 592 | 0.52 | 1.50 |
| NR-T145-CB | 5.06 | 406 | 0.63 | 2.88 |
| NR-T145-SWCNTs | 3.44 | 372 | 0.65 | 2.50 |
| Sample ID | Tear Strength (N m−1) | Hardness | g (g mol)−1 |
|---|---|---|---|
| NR-S | 68.6 | 30.4 | 7353 |
| NR-S-CB | 61.2 | 31.6 | 7353 |
| NR-S-SWCNTs | 71.4 | 33.6 | 9434 |
| NR-T29 | 6.3 | 17.2 | 64,831 |
| NR-T29-CB | 7.7 | 16.5 | 101,555 |
| NR-T29-SWCNTs | 8.0 | 18.2 | 17,752 |
| NR-DCP | 51.5 | 35.4 | 8571 |
| NR-DCP-CB | 38.2 | 37.6 | 108,042 |
| NR-DCP-SWCNTs | 55.1 | 37.4 | 7353 |
| NR-T145 | 36.2 | 23.3 | 19,584 |
| NR-T145-CB | 15.3 | 21.2 | 48,723 |
| NR-T145-SWCNTs | 23.4 | 24 | 29,215 |
| NR-SWCNTs Composites | ID/IG Ratio | Frequency, δ (cm−1) |
|---|---|---|
| SWCNTs (pristine) | 0.2452 | 0 |
| NR-S-SWCNTs | 0.3778 | 5 |
| NR-T29-SWCNTs | 0.3648 | 13 |
| NR-DCP-SWCNTs | 0.3195 | 16 |
| NR-T145-SWCNTs | 0.3520 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Martínez, Y.; Ramírez-Herrera, C.A.; Mondragón, M.; Elías-Zúñiga, A.; Elizalde, L.E. Effect of Single-Walled Carbon Nanotubes on the Cross-Linking Process in Natural Rubber Vulcanization. Polymers 2023, 15, 126. https://doi.org/10.3390/polym15010126
Vázquez-Martínez Y, Ramírez-Herrera CA, Mondragón M, Elías-Zúñiga A, Elizalde LE. Effect of Single-Walled Carbon Nanotubes on the Cross-Linking Process in Natural Rubber Vulcanization. Polymers. 2023; 15(1):126. https://doi.org/10.3390/polym15010126
Chicago/Turabian StyleVázquez-Martínez, Yoliria, Claudia A. Ramírez-Herrera, Margarita Mondragón, Alex Elías-Zúñiga, and Luis E. Elizalde. 2023. "Effect of Single-Walled Carbon Nanotubes on the Cross-Linking Process in Natural Rubber Vulcanization" Polymers 15, no. 1: 126. https://doi.org/10.3390/polym15010126
APA StyleVázquez-Martínez, Y., Ramírez-Herrera, C. A., Mondragón, M., Elías-Zúñiga, A., & Elizalde, L. E. (2023). Effect of Single-Walled Carbon Nanotubes on the Cross-Linking Process in Natural Rubber Vulcanization. Polymers, 15(1), 126. https://doi.org/10.3390/polym15010126