Degradation Behaviors and Mechanism of Nitrile Butadiene Rubber Caused by Insulating Medium C5F10O
Abstract
:1. Introduction
2. Experiment Method
2.1. Thermal Accelerated Ageing Method
2.2. Preparation for the NBR Samples
3. Deterioration Behaviors of NBR Aged in C5F10O/N2 Mixture
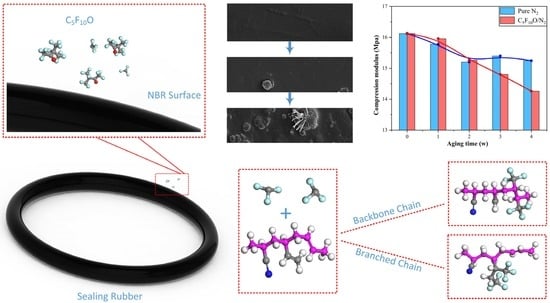
3.1. Compressive Modulus
3.2. Surface Morphology and Element Content of NBR
- I.
- Preliminary reaction. In the long-term contact with C5F10O, copolymer of butadiene and acrylonitrile starts to react with C5F10O, resulting in a decrease in the elasticity and strength on the partial surface. However, the effect is not significant in the initial stage, so the rubber surface remains smooth.
- II.
- Additive migration. As the surface of NBR continues deteriorating, it is easier for the reinforcing agent inside the rubber to come out of the less-strength surface during its immigration urged by heat. This is also why bulges wrapping ZnO or CaCO3 crystals were found on the NBR surface.
- III.
- Additive precipitation. As the elasticity and hardness of the rubber surface continue to decrease, more and more additives migrate to the surface of the rubber and then pierce the weakened surface, forming observable dot-like crystals. After that, the additives begin to extend on the damaged surface and finally form dendritic crystals on the protrusions, whose volume is also greatly enlarged. This process further promotes the deterioration of the rubber’s mechanical performance.
4. Deterioration Mechanism Analysis of NBR Aged in C5F10O/N2 Mixture
4.1. Reaction Mechanism of NBR and C5F10O
4.1.1. Primary Decomposition of C5F10O
4.1.2. C=C Double Bonds in NBR
4.2. Simulation of Rubber Mechanical Properties Based on Molecular Dynamics
4.2.1. Model Building
4.2.2. Deterioration Mechanism of NBR
5. Conclusions
- (1)
- The compressive modulus of NBR aged in C5F10O/N2 mixture is significantly smaller than that of NBR aged in N2, indicating that C5F10O is incompatible with NBR rubber. Therefore, when in long-term contact with C5F10O in the electrical equipment, the sealing performance and the service life of NBR will be weakened.
- (2)
- NBR aged in C5F10O undergoes a three-stage deterioration process based on changes in its surface morphology and atomic composition: (I) a preliminary reaction between NBR and C5F10O results in the reduction of surface strength; (II) reinforcing agents such as ZnO and CaCO3 inside the NBR migrate to the surface, forming bumps that encase crystals on the rubber surface; (III) with further weakening of the surface strength, the reinforcing agents penetrate the rubber surface and exhibit branch-like extensions at the bumps. Each stage is accompanied by a decrease in the mechanical strength of NBR.
- (3)
- DFT and MD simulations suggest that the C=C double bonds in the molecular chain of NBR can react with CF3 radicals generated by the primary decomposition of C5F10O. Then the addition of C=C double bonds and introduction of CF3 groups on the molecular chain will cause a decrease in the elastic, shear and bulk modulus of NBR. This results in the internal reinforcing agents precipitating onto the surface of NBR, thereby further intensifying the irreversible deterioration of its mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, F.; Li, H.; Cheng, H.; Tang, J.; Liu, Y. SF6 decomposition and insulation condition monitoring of GIE: A review. High Volt. 2021, 6, 955–966. [Google Scholar] [CrossRef]
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Rogelj, J.; Séférian, R.; Wartenburger, R.; Allen, M.R.; Cain, M.; Millar, R.J.; Ebi, K.L.; Ellis, N.; Hoegh-Guldberg, O.; et al. The many possible climates from the Paris Agreement’s aim of 1.5 °C warming. Nature 2018, 558, 41–49. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EU) No 517/2014 Of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32014R0517 (accessed on 20 May 2014).
- Stoller, P.C.; Doiron, C.B.; Tehlar, D.; Simka, P.; Ranjan, N. Mixtures of CO2 and C5F10O perfluoroketone for high voltage applications. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2712–2721. [Google Scholar] [CrossRef]
- Zhong, J.; Fu, X.; Yang, A.; Han, G.; Liu, J.; Lu, Y.; Wang, X.; Rong, M. Insulation performance and liquefaction characteristic of C5F10O/CO2 gas mixture. In Proceedings of the 2017 4th International Conference on Electric Power Equipment-Switching Technology, Xian, China, 22–25 October 2017; pp. 291–294. [Google Scholar]
- Li, X.; Deng, Y.; Jiang, X.; Zhao, H.; Zhuo, R.; Wang, D.B.; Fu, M.L. Insulation performance and application of enviroment-friendly gases mixtures of C4F7N and C5F10O with CO2. High Volt. Eng. 2017, 43, 708–714. [Google Scholar]
- Zeng, F.; Su, D.; Chen, X.; Xie, B.; Zhang, S.; Yao, Q.; Tang, J. Switching impulse characteristics of C5F10O gas mixtures under extremely nonuniform field. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1617–1624. [Google Scholar] [CrossRef]
- She, C.; Zeng, F.; Dai, L.; Tang, B.; Yao, Q.; Li, L.; Tang, J. Self-recovery pathways of C5F10O after over thermal decomposition. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1450–1458. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, S.; Li, X. Chemical kinetics of C5F10O with reactive center dot OH radical induced in AOP in gaseous and aqueous phases. Plasma Chem. Plasma Process. 2022, 42, 1265–1278. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Li, X. Decomposition pathway and kinetic analysis of perfluoroketone C5F10O. J. Phys. D Appl. Phys. 2020, 53, 415502. [Google Scholar] [CrossRef]
- Zeng, F.; Wan, Z.; Lei, Z.; Tang, J.; Dai, L.; Wang, X.; Yao, Q. Over thermal decomposition characteristics of C5F10O: An environmentally friendly insulation medium. IEEE Access 2019, 7, 62080–62086. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, D.; Li, Y.; Zhang, J.; Cui, Z.; Xiao, S.; Tang, J. Theoretical study on the interaction between C5-PFK and Al (111), Ag (111): A comparative study. Appl. Surf. Sci. 2018, 464, 586–596. [Google Scholar] [CrossRef]
- Zeng, F.; Feng, X.; Lei, Z.; Xia, Y.; Wu, S.; Zhang, S.; Yao, Q.; Tang, J. Thermal decomposition mechanism of environmental-friendly insulating gas C5F10O on Cu (111) surface. Plasma Chem. Plasma Process. 2021, 41, 1455–1469. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, Y.; Tang, F.; Lv, Q.; Zhang, J.; Xiao, S.; Tang, J.; Zhang, X. Experimental study on compatibility of eco-friendly insulating medium C5F10O/CO2 gas mixture with copper and aluminum. IEEE Access 2019, 7, 83994–84002. [Google Scholar] [CrossRef]
- Minagawa, T.; Yasuda, M. Accelerated ageing tests of rubber O-rings for gas-insulated substation equipment. Kagaku Kogaku Ronbunshu 2019, 45, 204–210. [Google Scholar] [CrossRef]
- She, C.; Tang, J.; Cai, R.; Li, H.; Li, L.; Yao, Q.; Zeng, F.; Li, C. Compatibility of C5F10O with common-used sealing materials: An experimental study. AIP Adv. 2021, 11, 065220. [Google Scholar] [CrossRef]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green gas to replace SF6 in electrical grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Stuckless, H.A.; Braun, J.M.; Chu, F.Y. Degradation of silica-filled epoxy spacers by ARC contaminated gases in SF6-insulated equipment. IEEE Trans. Power Appar. Syst. 1986, 104, 3597–3602. [Google Scholar]
- Wu, H.; Zhao, Y.; Su, L.; Wang, K.; Dong, X.; Wang, D. Markedly improved photo-oxidation stability of alpha form isotactic polypropylene with nodular morphology. Polym. Degrad. Stab. 2021, 189, 109595. [Google Scholar] [CrossRef]
- Gao, W.; Cao, Y.; Wang, Y.; Price, C.; Ronzello, J.; Uzelac, N.; Laso, A.; Tefferi, M.; Darko, K. Materials compatibility study of C4F7N/CO2 gas mixture for medium-voltage switchgear. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 270–278. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, P.; Cheng, L.; Liang, S. Compatibility and Interaction Mechanism between EPDM Rubber and a SF6 alternative gas-C4F7N/CO2/O2. ACS Omiga 2021, 6, 13293–13299. [Google Scholar] [CrossRef]
- ISO/188-2011; Rubber, Vulcanized or Thermoplastic—Accelerated Ageing and Heat Resistance Tests. International Organization for Standardization: Geneva, Switzerland, 2011.
- BS EN ISO 11114-2-2013; Gas Cylinders—Compatibility of Cylinder and Valve Materials with Gas Contents. Compressed Gas Association: McLean, VA, USA, 2013.
- Woo, C.S.; Choi, S.S.; Lee, S.B.; Kim, H.S. Useful lifetime prediction of rubber components using accelerated testing. IEEE Trans. Reliab. 2010, 59, 11–17. [Google Scholar] [CrossRef]
- ISO/7743-2018; Rubber, Vulcanized or Thermoplastic–Determination of Compression Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2018.
- Loadman, M.J. Analysis of Rubber and Rubber-like Polymers; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Zhong, L.; Deng, Y.; Liu, J.; Wang, F.; Chen, S.; Sun, Q.; Duan, X.; Huang, H. Theoretical study by density functional theory calculations of decomposition processes and primary products of C5F10O with moisture content. J. Phys. D Appl. Phys. 2020, 53, 485204. [Google Scholar] [CrossRef]
- Fu, Y.; Rong, M.; Wang, X.; Yang, A. Rate constants of C5F10O decomposition reactions at temperatures of 300–3500 K. J. Phys. D Appl. Phys. 2019, 52, 035202. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Tian, S.; Xiao, S.; Chen, D.; Tang, J.; Zhuo, R. Decomposition mechanism of the C5-PFK/CO2 gas mixture as an alternative gas for SF6. Chem. Eng. J. 2018, 336, 38–46. [Google Scholar] [CrossRef]
- Beeke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1998, 98, 5648–5652. [Google Scholar] [CrossRef]
- Savin, A.V.; Mazo, M.A. The COMPASS force field: Validation for carbon nanoribbons. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 118, 113937. [Google Scholar] [CrossRef]
- Saha, S.; Bhowmick, A.K. An Insight into molecular structure and properties of flexible amorphous polymers: A molecular dynamics simulation approach. J. Appl. Polym. Sci. 2019, 136, 47457. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, X.; Shi, R.; Zhang, H.; Han, R.; Cheng, X.; Zhou, C. Tetraphenylphenyl-modified damping additives for silicone rubber: Experimental and molecular simulation investigation. Mater. Des. 2021, 202, 109551. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Wang, Q.; Li, X.; Hao, J. Selection of optimum polymerization degree and force field in the molecular dynamics simulation of insulating paper cellulose. Energies 2017, 10, 1377. [Google Scholar] [CrossRef]
- Du, D.; Tang, C.; Tang, Y.; Yang, L.; Hao, J. Molecular simulation on the mechanical and thermal properties of carbon nanowire modified cellulose insulating paper. Compos. Struct. 2020, 261, 113283. [Google Scholar] [CrossRef]
- Theodorou, D.N.; Suter, U.W. Atomistic modeling of mechanical properties of polymeric glasses. Macromolecules 1985, 19, 139–154. [Google Scholar] [CrossRef]





 C,
C,  O,
O,  F;
F;  C in backbone,
C in backbone,  H,
H,  N. The sequence in the repeat unit is acrylonitrile, 1,2-addition butadiene, 1,4-addition butadiene.)
N. The sequence in the repeat unit is acrylonitrile, 1,2-addition butadiene, 1,4-addition butadiene.)
 C,
C,  O,
O,  F;
F;  C in backbone,
C in backbone,  H,
H,  N. The sequence in the repeat unit is acrylonitrile, 1,2-addition butadiene, 1,4-addition butadiene.)
N. The sequence in the repeat unit is acrylonitrile, 1,2-addition butadiene, 1,4-addition butadiene.)




| Composition | Weight Fraction (%) | Temperature (°C) |
|---|---|---|
| Oil | 5.8 | 200~380 |
| Polymer | 49.0 | 390~500 |
| Carbon black | 39.8 | 527~600 |
| CaCO3 | 0.38 | 610~637 |
| ZnO/MgO | 4.14 | >800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
She, C.; Zeng, F.; Dai, L.; Li, L.; Yao, Q.; Tang, J. Degradation Behaviors and Mechanism of Nitrile Butadiene Rubber Caused by Insulating Medium C5F10O. Polymers 2023, 15, 2282. https://doi.org/10.3390/polym15102282
She C, Zeng F, Dai L, Li L, Yao Q, Tang J. Degradation Behaviors and Mechanism of Nitrile Butadiene Rubber Caused by Insulating Medium C5F10O. Polymers. 2023; 15(10):2282. https://doi.org/10.3390/polym15102282
Chicago/Turabian StyleShe, Congdong, Fuping Zeng, Liangjun Dai, Long Li, Qiang Yao, and Ju Tang. 2023. "Degradation Behaviors and Mechanism of Nitrile Butadiene Rubber Caused by Insulating Medium C5F10O" Polymers 15, no. 10: 2282. https://doi.org/10.3390/polym15102282
APA StyleShe, C., Zeng, F., Dai, L., Li, L., Yao, Q., & Tang, J. (2023). Degradation Behaviors and Mechanism of Nitrile Butadiene Rubber Caused by Insulating Medium C5F10O. Polymers, 15(10), 2282. https://doi.org/10.3390/polym15102282