Preparation and Application of a Multifunctional Interfacial Modifier for Ramie Fiber/Epoxy Resin Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals Used
2.2. Synthesis of 4,4′-(9H-fluorene-9,9-diyl) Dianilineand 6,6′-((((9H-fluorene-9,9-diyl)bis(4,1-phenylene))bis(azanediyl))bis((3,4-dihydroxyphenyl)methylene)) Bis (dibenzo (c,e)(1,2)oxaphosphinine 6-oxide) (FPD)
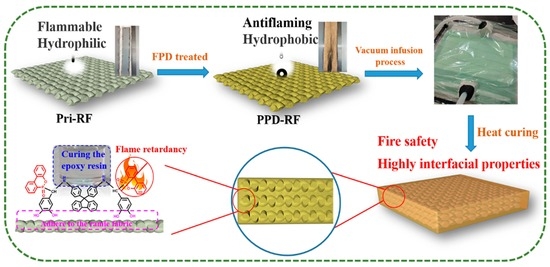
2.3. Fabrication of FPD on Ramie Fabric by Mussel Inspiration
2.4. Preparation of Ramie-Epoxy Resin Composites
2.5. Characterization
3. Results and Discussion
3.1. Synthesis of FPD and Characterization of Mussel-Inspired Ramie Fabric
3.2. Mechanical Properties and Compatibility
3.3. Thermal Stability and Flame Resistance Performance of Composites
3.4. Flame-Retardant Mechanism
3.4.1. The Analysis of Thermal Degradation Gaseous Products
3.4.2. The Characteristic of the Condensed Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Yu, T.; Cong, L. Reinforcement of denture base resin with short vegetable fiber. Dent. Mater. 2013, 29, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xian, G.; Yi, X.-S. Life Cycle Assessment of Ramie Fiber Used for FRPs. Aerospace 2018, 5, 81. [Google Scholar] [CrossRef]
- Houston, J.; Houston, D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Inuwa, I.M.; Hassan, A. A review on potential development of flame retardant kenaf fibers reinforced polymer composites. Polym. Adv. Technol. 2017, 28, 424–434. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Baruah, P.; Karak, N. Bio-based tough hyperbranched epoxy/graphene oxide nanocomposite with enhanced biodegradability attribute. Polym. Degrad. Stab. 2016, 129, 26–33. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Subasinghe, A.; Bhattacharyya, D. Performance of different intumescent ammonium polyphosphate flame retardants in PP/kenaf fibre composites. Compos. Part A Appl. Sci. Manuf. 2014, 65, 91–99. [Google Scholar] [CrossRef]
- Rybyan, A.A.; Bilichenko, J.V.; Kireev, V.V.; Kolenchenko, A.A.; Chistyakov, E.M. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers 2022, 14, 5334. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. State of the art and perspectives on sol–gel derived hybrid architectures for flame retardancy of textiles. J. Mater. Chem. 2012, 22, 21805–21809. [Google Scholar] [CrossRef]
- Tai, Q.; Song, L.; Hu, Y.; Yuen, R.K.K.; Feng, H.; Tao, Y. Novel styrene polymers functionalized with phosphorus–nitrogen containing molecules: Synthesis and properties. Mater. Chem. Phys. 2012, 134, 163–169. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Batool, S.; Song, L.; Hu, Y. A phosphaphenanthrene-containing vanillin derivative as co-curing agent for flame-retardant and antibacterial epoxy thermoset. Polymer 2021, 217, 123460. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Yu, X.; Xu, J.; Pan, Z.; Zhou, H. Reactive organophosphorus flame retardant for transparency, low-flammability, and mechanical reinforcement epoxy resin. J. Appl. Polym. Sci. 2021, 138, 50536. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Yousif, B.F.; Ku, H. Suitability of using coir fiber/polymeric composite for the design of liquid storage tanks. Mater. Des. 2012, 36, 847–853. [Google Scholar] [CrossRef]
- Shumao, L.; Jie, R.; Hua, Y.; Tao, Y.; Weizhong, Y. Influence of ammonium polyphosphate on the flame retardancy and mechanical properties of ramie fiber-reinforced poly(lactic acid) biocomposites. Polym. Int. 2010, 59, 242–248. [Google Scholar] [CrossRef]
- Dittenber, D.B.; GangaRao, H.V.S. Critical review of recent publications on use of natural composites in infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef]
- Tang, X.-Z.; Yu, B.; Hansen, R.V.; Chen, X.; Hu, X.; Yang, J. Grafting Low Contents of Branched Polyethylenimine onto Carbon Fibers to Effectively Improve Their Interfacial Shear Strength with an Epoxy Matrix. Adv. Mater. Interfaces 2015, 2, 1500122. [Google Scholar] [CrossRef]
- Chhetri, S.; Bougherara, H. A comprehensive review on surface modification of UHMWPE fiber and interfacial properties. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106146. [Google Scholar] [CrossRef]
- Xing, L.; Liu, L.; Huang, Y.; Jiang, D.; Jiang, B.; He, J. Enhanced interfacial properties of domestic aramid fiber-12 via high energy gamma ray irradiation. Compos. Part B Eng. 2015, 69, 50–57. [Google Scholar] [CrossRef]
- Shih, Y.-F. Mechanical and thermal properties of waste water bamboo husk fiber reinforced epoxy composites. Mat. Sci. Eng. A 2007, 445–446, 289–295. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Wei, J.; Wu, H. Nondestructive Modification of Catechol/Polyethyleneimine onto Polyester Fabrics by Mussel-Inspiration for Improving Interfacial Performance. Macromol. Mater. Eng. 2020, 305, 2000258. [Google Scholar] [CrossRef]
- Seki, Y. Innovative multifunctional siloxane treatment of jute fiber surface and its effect on the mechanical properties of jute/thermoset composites. Mat. Sci. Eng. A 2009, 508, 247–252. [Google Scholar] [CrossRef]
- Akram Bhuiyan, M.S.; Roland, J.D.; Liu, B.; Reaume, M.; Zhang, Z.; Kelley, J.D.; Lee, B.P. In Situ Deactivation of Catechol-Containing Adhesive Using Electrochemistry. J. Am. Chem. Soc. 2020, 142, 4631–4638. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.; Zeng, H.; Xu, Z.K. Deposition and Adhesion of Polydopamine on the Surfaces of Varying Wettability. ACS Appl. Mater. Interf. 2017, 9, 30943–30950. [Google Scholar] [CrossRef]
- Ryou, M.H.; Lee, Y.M.; Park, J.K.; Choi, J.W. Mussel-inspired polydopamine-treated polyethylene separators for high-power li-ion batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, M.Q.; Chen, T.T.; Zhang, H.; Hu, D.F.; Wu, B.H.; Ji, J.; Xu, Z.K. Dopamine-Triggered One-Step Polymerization and Codeposition of Acrylate Monomers for Functional Coatings. ACS Appl. Mater. Interf. 2017, 9, 34356–34366. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busque, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. Engl. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kang, L.; Yue, Z.; Liu, X.; Wallace, G.G. Composite Tissue Adhesive Containing Catechol-Modified Hyaluronic Acid and Poly-l-lysine. ACS Appl. Bio Mater. 2019, 3, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Teng, N.; Peng, Y.; Liu, Y.; Cao, L.; Zhu, J.; Liu, X. Biobased Benzoxazine Derived from Daidzein and Furfurylamine: Microwave-Assisted Synthesis and Thermal Properties Investigation. ChemSusChem 2018, 11, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, N.; Cheng, J.; Song, P.; Fang, Z.; Wang, H. Fabrication of flame retardant benzoxazine semi-biocomposites reinforced by ramie fabrics with bio-based flame retardant coating. Polym. Compos. 2018, 39, E480–E488. [Google Scholar] [CrossRef]
- Song, X.; Song, F.; Ding, X.-M.; Wu, J.-M.; Wang, X.-H.; Wang, F.; Feng, R.; Wang, X.-L.; Wang, Y.-Z. Construction of bio-based ramie fabric/epoxy resin composites with high flame retardant and mechanical performances. Ind. Crop. Prod. 2023, 194, 116281. [Google Scholar] [CrossRef]
- Kwak, W.G.; Oh, M.H.; Gong, M.S. Preparation of silver-coated cotton fabrics using silver carbamate via thermal reduction and their properties. Carbohydr. Polym. 2015, 115, 317–324. [Google Scholar] [CrossRef]
- Tian, C.; Wang, C.; Ren, X.; Hong, L. Synthesis of silane-modified polyphosphate esters and its application in transparent flame-retardant coatings. J. Appl. Polym. Sci. 2019, 136, 47199. [Google Scholar] [CrossRef]
- Luo, Y.F.; Wang, S.; Du, X.S.; Du, Z.L.; Cheng, X.; Wang, H.B. Durable flame retardant and water repellent cotton fabric based on synergistic effect of ferrocene and DOPO. Cellulose 2021, 28, 1809–1826. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. An environmentally friendly approach to fabricating flame retardant, antibacterial and antifungal cotton fabrics via self-assembly of guanazole-metal complex. J. Clean. Prod. 2020, 273, 122832. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.; Wang, S.; Hu, J.; Liu, X. Hyperbranched flame retardant for epoxy resin modification: Simultaneously improved flame retardancy, toughness and strength as well as glass transition temperature. Chem. Eng. J. 2022, 428, 131226. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Yin, G.-Z.; Wang, D.-Y. Construction of a novel three-in-one biomass based intumescent fire retardant through phosphorus functionalized metal-organic framework and β-cyclodextrin hybrids in achieving fire safe epoxy. Compos. Commun. 2021, 23, 100594. [Google Scholar] [CrossRef]










| Samples | Ramie Fiber (g) | DER-331 (g) | DDM (g) | FPD (g) | Content of P (wt.%) |
|---|---|---|---|---|---|
| RFEPC-0 | 120 | 120 | 30.3 | 0 | 0 |
| RFEPC-1 | 120 | 120 | 29.6 | 12 | 1.70 |
| RFEPC-2 | 120 | 120 | 28.9 | 24 | 3.38 |
| RFEPC-3 | 120 | 120 | 28.2 | 36 | 5.04 |
| Sample | Tensile | Flexural | Impact Strength (kJ·m−2) | ||
|---|---|---|---|---|---|
| Strength (MPa) | Modulus (MPa) | Strength (MPa) | Modulus (MPa) | ||
| RFEPC-0 | 70 ± 16 | 4212 ± 776 | 107 ± 7 | 4670 ± 276 | 5.61 ± 0.27 |
| RFEPC-1 | 74.8 ± 3 | 5979 ± 207 | 111 ± 2 | 5020 ± 688 | 6.17 ± 0.21 |
| RFEPC-2 | 83 ± 2.4 | 6248 ± 435 | 112 ± 2.2 | 5390 ± 252 | 6.83 ± 0.15 |
| RFEPC-3 | 96 ± 13 | 6779 ± 563 | 116 ± 6. 7 | 5570 ± 322 | 7.22 ± 0.24 |
| Samples | Td10 (°C) | Char Yield at 800 °C (%) | Rmax (%/°C) | LOI (%) | t1/t2 (s) | Dripping | UL-94 |
|---|---|---|---|---|---|---|---|
| RFEPC-0 | 342.3 | 9.92 | 398.7 | 23.4 | - a | N | No-rating |
| RFEPC-1 | 329.3 | 16.84 | 399 | 26.8 | - a | N | No-rating |
| RFEPC-2 | 318.3 | 22.05 | 353 | 29.6 | - a | N | No-rating |
| RFEPC-3 | 272.3 | 27.77 | 309 | 34.6 | 2.3/5.6 | N | V-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, J.; Dai, J.; Zhang, X.; Liu, X.; Liu, X.; Yi, X. Preparation and Application of a Multifunctional Interfacial Modifier for Ramie Fiber/Epoxy Resin Composites. Polymers 2023, 15, 3800. https://doi.org/10.3390/polym15183800
Zhang L, Liu J, Dai J, Zhang X, Liu X, Liu X, Yi X. Preparation and Application of a Multifunctional Interfacial Modifier for Ramie Fiber/Epoxy Resin Composites. Polymers. 2023; 15(18):3800. https://doi.org/10.3390/polym15183800
Chicago/Turabian StyleZhang, Liyue, Jingkai Liu, Jinyue Dai, Xufeng Zhang, Xiaoling Liu, Xiaoqing Liu, and Xiaosu Yi. 2023. "Preparation and Application of a Multifunctional Interfacial Modifier for Ramie Fiber/Epoxy Resin Composites" Polymers 15, no. 18: 3800. https://doi.org/10.3390/polym15183800
APA StyleZhang, L., Liu, J., Dai, J., Zhang, X., Liu, X., Liu, X., & Yi, X. (2023). Preparation and Application of a Multifunctional Interfacial Modifier for Ramie Fiber/Epoxy Resin Composites. Polymers, 15(18), 3800. https://doi.org/10.3390/polym15183800