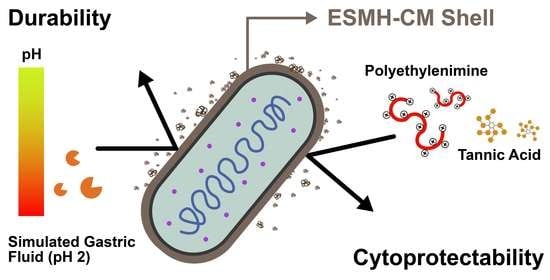
Cytoprotection of Probiotic Lactobacillus acidophilus with Artificial Nanoshells of Nature-Derived Eggshell Membrane Hydrolysates and Coffee Melanoidins in Single-Cell Nanoencapsulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. One-Step Formation of ESMH-CM Films and Shells on Abiotic Substrates
2.2. Single-Cell Nanoencapsulation (SCNE) and Characterizations
3. Results and Discussion
3.1. One-Step Formation of ESMH-CM Films and Shells on Abiotic Substrates
3.2. One-Step SCNE of S. cerevisiae
3.3. One-Step SCNE of Probiotic L. acidophilus and L. brevis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | poly(acrylic acid) |
| AFM | atomic force microscopy |
| CaCl2 | calcium chloride |
| CaCO3 | calcium carbonate |
| CLSM | confocal laser-scanning microscopy |
| CM | coffee melanoidin |
| DMSO | dimethylsulfoxide |
| ESMH | eggshell membrane hydrolysate |
| FDA | fluorescein diacetate |
| FE-SEM | field-emission scanning electron microscopy |
| FT-IR | Fourier-transform infrared |
| GI | gastrointestinal |
| LbL | layer-by-layer |
| NaCl | sodium chloride |
| Na2CO3 | sodium carbonate |
| PC | polycarbonate |
| PE | polyethylene |
| PEI | polyethylenimine |
| PI | propidium iodide |
| PSS | poly(sodium 4-styrenesulfonate) |
| PTFE | polytetrafluoroethylene |
| PU | polyurethane |
| PVPON | poly(N-vinylpyrrolidone) |
| SCNE | single-cell nanoencapsulation |
| SGF | simulated gastric fluid |
| SS | stainless steel |
| SiO2 | Silica |
| TA | tannic acid |
| TAMRA | carboxytetramethylrhodamine |
| YPD | yeast-extract-peptone-dextrose |
| XPS | X-ray photoelectron spectroscopy |
References
- Oliveira, M.B.; Hatami, J.; Mano, J.F. Coating strategies using layer-by-layer deposition for cell encapsulation. Chem. Asian J. 2016, 11, 1753–1764. [Google Scholar] [CrossRef] [Green Version]
- Fakhrullin, R.F.; Lvov, Y.M. “Face-lifting” and “make-up” for microorganisms: Layer-by-layer polyelectrolyte nanocoating. ACS Nano 2012, 6, 4557–4564. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Zhung, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical applications of layer-by-layer self-assembly for cell encapsulation: Current status and future perspective. Adv. Healthcare Mater. 2019, 8, 1800939. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in layer-by-layer assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [Green Version]
- Yun, G.; Kang, D.G.; Rheem, H.B.; Lee, H.; Han, S.Y.; Park, J.; Cho, W.K.; Han, S.M.; Choi, I.S. Reversed anionic Hofmeister effect in metal-phenolic-based film formation. Langmuir 2020, 36, 15552–15557. [Google Scholar] [CrossRef]
- Yun, G.; Youn, W.; Lee, H.; Han, S.Y.; Oliveira, M.B.; Cho, H.; Caruso, F.; Mano, J.F.; Choi, I.S. Dynamic electrophoretic assembly of metal-phenolic films: Accelerated formation and cytocompatible detachment. Chem. Mater. 2020, 32, 7746–7753. [Google Scholar] [CrossRef]
- Yun, G.; Richardson, J.J.; Capelli, M.; Hu, Y.; Besford, Q.A.; Weiss, A.C.G.; Lee, H.; Choi, I.S.; Gibson, B.C.; Reineck, P.; et al. The biomolecular corona in 2D and reverse: Patterning metal-phenolic networks on proteins, lipids, nucleic acids, polysaccharides, and fingerprints. Adv. Funct. Mater. 2020, 30, 1905805. [Google Scholar] [CrossRef]
- Yun, G.; Richardson, J.J.; Biviano, M.; Caruso, F. Tuning the mechanical behavior of metal-phenolic networks through building block composition. ACS Appl. Mater. Interfaces 2019, 11, 6404–6410. [Google Scholar] [CrossRef]
- Yun, G.; Besford, Q.A.; Johnston, S.T.; Richardson, J.J.; Pan, S.; Biviano, M.; Caruso, F. Self-assembly of nano- to macroscopic metal-phenolic materials. Chem. Mater. 2018, 30, 5750–5758. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.I.; Youn, W.; Park, T.; Lee, S.; Kim, T.-S.; Mano, J.F.; Choi, I.S. Iron gall ink revisited: In situ oxidation of Fe(II)-tannin complex for fluidic-interface engineering. Adv. Mater. 2018, 30, 1805091. [Google Scholar] [CrossRef]
- Han, S.Y.; Kang, E.K.; Choi, I.S. Iron gall ink revisited: A surfactant-free emulsion technology for black hair-dyeing formulation. Cosmetics 2021, 8, 9. [Google Scholar] [CrossRef]
- Han, S.Y.; Hong, S.-P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron gall ink revisited: Natural formulation for black hair-dyeing. Cosmetics 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Park, J.; Han, S.Y.; Han, S.; Youn, W.; Choi, H.; Yun, G.; Choi, I.S. Ascorbic acid-mediated reductive disassembly of Fe3+-tannic acid shells in degradable single-cell nanoencapsulation. Chem. Commun. 2020, 56, 13748–13751. [Google Scholar] [CrossRef]
- Lee, H.; Nguyen, D.T.; Kim, N.; Han, S.Y.; Hong, Y.J.; Yun, G.; Kim, B.J.; Choi, I.S. Enzyme-mediated kinetic control of Fe3+-tannic acid complexation for interface engineering. ACS Appl. Mater. Interfaces 2021, 13, 52385–52394. [Google Scholar] [CrossRef]
- Youn, W.; Kim, J.Y.; Park, J.; Kim, N.; Choi, H.; Cho, H.; Choi, I.S. Single-cell nanoencapsulation: From passive to active shells. Adv. Mater. 2020, 32, 1907001. [Google Scholar] [CrossRef]
- Kim, B.J.; Cho, H.; Park, J.H.; Mano, J.F.; Choi, I.S. Strategic advances in formation of cell-in-shell structures: From syntheses to applications. Adv. Mater. 2018, 30, 1706063. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, D.; Lee, J.; Choi, I.S. Cell-in-shell hybrids: Chemical nanoencapsulation of individual cells. Acc. Chem. Res. 2016, 49, 792–800. [Google Scholar] [CrossRef]
- Park, J.H.; Yang, S.H.; Lee, J.; Ko, E.H.; Hong, D.; Choi, I.S. Nanocoating of single cells: From maintenance of cell viability to manipulation of cellular activities. Adv. Mater. 2014, 26, 2001–2010. [Google Scholar] [CrossRef]
- Hong, D.; Park, M.; Yang, S.H.; Lee, J.; Kim, Y.-G.; Choi, I.S. Artificial spores: Cytoprotective nanoencapsulation of living cells. Trends Biotechnol. 2013, 31, 442–447. [Google Scholar] [CrossRef]
- Yang, S.H.; Hong, D.; Lee, J.; Ko, E.H.; Choi, I.S. Artificial spores: Cytocompatible encapsulation of individual living cells within thin, though artificial shells. Small 2013, 9, 178–186. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Z.; Fu, X.; Du, L.; Tian, Y.; Wang, J.; Cai, W.; Guo, P.; Wu, C. Synthesis of a removable cytoprotective exoskeleton by tea polyphenol complexes for living cell encapsulation. ACS Biomater. Sci. Eng. 2021, 7, 764–771. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Harbaugh, S.; Drachuk, I.; Shchepelina, O.; Kelley-Loughnane, N.; Stone, M.; Tsukruk, V.V. Hydrogen-bonded LbL shells for living cell surface engineering. Soft Matter 2011, 7, 2364–2372. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Choi, I.S.; Lvov, Y.M. (Eds.) Cell Surface Engineering: Fabrication of Functional Nanoshells; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Kim, S.; Youn, W.; Choi, I.S.; Park, J.H. Thickness-tunable eggshell membrane hydrolysate nanocoating with enhanced cytocompatibility and neurite outgrowth. Langmuir 2019, 35, 12562–12568. [Google Scholar] [CrossRef]
- Han, S.Y.; Lee, H.; Nguyen, D.T.; Yun, G.; Kim, S.; Park, J.H.; Choi, I.S. Single-cell nanoencapsulation of Saccharomyces cerevisiae by cytocompatible layer-by-layer assembly of eggshell membrane hydrolysate and tannic acid. Adv. NanoBiomed Res. 2021, 1, 2000037. [Google Scholar] [CrossRef]
- Han, S.Y.; Yun, G.; Nguyen, D.T.; Kang, E.K.; Lee, H.; Kim, S.; Kim, B.J.; Park, J.H.; Choi, I.S. Hydrogen bonding-based layer-by-layer assembly of nature-derived eggshell membrane hydrolysates and coffee melanoidins in single-cell nanoencapsulation. ChemNanoMat 2022, 8, e202100535. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Baláž, M. Eggshell membrane biomaterials as a platform for applications in material science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Chen, X.E.; Mangindaan, D.; Chien, H.-W. Green sustainable photothermal materials by spent coffee grounds. J. Taiwan Inst. Chem. Eng. 2022, 137, 104259. [Google Scholar] [CrossRef]
- Chien, H.-W.; Chen, X.-E. Spent coffee grounds as potential green photothermal materials for biofilm elimination. J. Environ. Chem. Eng. 2022, 10, 107131. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Iriondo-Dehond, A.; Casas, A.R.; Castillo, M.D. Interest of coffee melanoidins as sustainable healthier food ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.; Han, S.; Han, S.Y.; Passos, C.P.; Seo, J.; Lee, H.; Kang, E.K.; Mano, J.F.; Coimbra, M.A.; et al. Coffee melanoidin-based multipurpose film formation: Application to single-cell nanoencapsulation. ChemNanoMat 2020, 6, 379–385. [Google Scholar] [CrossRef]
- Ray, P.G.; Pal, P.; Srivas, P.K.; Basak, P.; Roy, S.; Dhara, S. Surface modification of eggshell membrane with electrospun chitosan/polycaprolactone nanofibers for enhanced dermal wound healing. ACS Appl. Bio Mater. 2018, 1, 985–998. [Google Scholar]
- Drachuk, I.; Gupta, M.K.; Tsukruk, V.V. Biomimetic coatings to control cellular function through cell surface engineering. Adv. Funct. Mater. 2013, 23, 4437–4453. [Google Scholar] [CrossRef]
- Chong, L.S.H.; Zhang, J.; Bhat, K.S.; Yong, D.; Song, J. Bioinspired cell-in-shell systems in biomedical engineering and beyond: Comparative overview and prospects. Biomaterials 2021, 266, 120473. [Google Scholar] [CrossRef]
- Altamirano-Ríos, A.V.; Guadarrama-Lezama, A.Y.; Arroyo-Maya, I.J.; Hernández-Álvarez, A.-J.; Orozco-Villafuerte, J. Effect of encapsulation methods and materials on the survival and viability of Lactobacillus acidophilus: A review. Int. J. Food Sci. Technol. 2022, 57, 4027–4040. [Google Scholar] [CrossRef]
- Gu, Q.; Yin, Y.; Yan, X.; Liu, X.; Liu, F.; McClements, D.J. Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: A review. Adv. Colloid Interface Sci. 2022, 309, 102781. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Coorey, R. A review on probiotic microencapsulation and recent advances of their application in bakery products. Food Bioprocess Technol. 2022, 15, 1677–1699. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [Green Version]
- Sbehat, M.; Mauriello, G.; Altamimi, M. Microencapsulation of probiotics for food functionalization: An update on literature reviews. Microorganisms 2022, 10, 1948. [Google Scholar] [CrossRef]
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for probiotic delivery. ACS Nano 2021, 15, 18653–18660. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control. Release 2022, 349, 184–205. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.; Lee, J.; Choi, J.Y.; Hong, D.; Yang, S.H.; Caruso, F.; Lee, Y.; Choi, I.S. A cytoprotective and degradable metal-polyphenol nanoshell for single-cell encapsulation. Angew. Chem. Int. Ed. 2014, 53, 12420–12425. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Choi, J.; Kim, D.; Hong, D.; Park, J.H.; Yang, S.H.; Choi, I.S. Chemical sporulation and germination: Cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid-FeIII complex. Nanoscale 2015, 7, 18918–18922. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Han, S.; Lee, K.-B.; Choi, I.S. Biphasic supramolecular self-assembly of ferric ions and tannic acid across interfaces for nanofilm formation. Adv. Mater. 2017, 29, 1700784. [Google Scholar] [CrossRef]
- Fan, G.; Wasuwanich, P.; Rodriguez-Otero, M.R.; Furst, A.L. Protection of anaerobic microbes from processing stressors using metal-phenolic networks. J. Am. Chem. Soc. 2022, 144, 2438–2443. [Google Scholar] [CrossRef]
- Pan, J.; Gong, G.; Wang, Q.; Shang, J.; He, Y.; Catania, C.; Birnbaum, D.; Li, Y.; Jia, Z.; Zhang, Y.; et al. A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea. Nat. Commun. 2022, 13, 2117. [Google Scholar] [CrossRef]
- Centurion, F.; Merhebi, S.; Baharfar, M.; Abbasi, R.; Zhang, C.; Mousavi, M.; Xie, W.; Yang, J.; Cao, Z.; Allioux, F.-M.; et al. Cell-mediated biointerfacial phenolic assembly for probiotic nano encapsulation. Adv. Funct. Mater. 2022, 32, 2200775. [Google Scholar] [CrossRef]
- Ma, D.-X.; Zhou, Y.; Wu, L.-D.; Li, Z.-Y.; Jiang, W.-J.; Huang, S.-L.; Guo, X.-P.; Sheng, J.-Z.; Wang, F.-S. Enhanced stability and function of probiotic Streptococcus thermophilus with self-encapsulation by increasing the biosynthesis of hyaluronan. ACS Appl. Mater. Interfaces 2022, 14, 42963–42975. [Google Scholar] [CrossRef]
- Wei, H.; Yang, X.-Y.; Geng, W.; van der Mei, H.C.; Busscher, H.J. Interfacial interactions between protective, surface-engineered shells and encapsulated bacteria with different cell surface composition. Nanoscale 2021, 13, 7220–7233. [Google Scholar] [CrossRef]
- Yuan, L.; Wei, H.; Yang, X.-Y.; Geng, W.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Escherichia coli colonization of intestinal epithelial layers in vitro in the presence of encapsulated Bifidobacterium breve for its protection against gastrointestinal fluids and antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 15973–15982. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A.M. Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef]
- Sbehat, M.; Altamimi, M.; Sabbah, M.; Mauriello, G. Layer-by-layer coating of single-cell Lacticaseibacillus rhamnosus to increase viability under simulated gastrointestinal conditions and use in film formation. Front. Microbiol. 2022, 13, 838416. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, M.; Wang, Y.; Wu, H.; Xiong, L.; Sun, Q. Enhanced viability of layer-by-layer encapsulated Lactobacillus pentosus using chitosan and sodium phytate. Food Chem. 2019, 285, 260–265. [Google Scholar] [CrossRef]
- Li, S.; Fan, L.; Li, S.; Sun, X.; Di, Q.; Zhang, H.; Li, B.; Liu, X. Validation of layer-by-layer coating as a procedure to enhance Lactobacillus plantarum survival during in vitro digestion, storage, and fermentation. J. Agric. Food Chem. 2023, 71, 1701–1712. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [Green Version]
- Ou, F.; McGoverin, C.; Swift, S.; Vanholsbeeck, F. Rapid and cost-effective evaluation of bacterial viability using fluorescence spectroscopy. Anal. Bioanal. Chem. 2019, 411, 3653–3663. [Google Scholar] [CrossRef] [Green Version]
- Buysschaert, B.; Byloos, B.; Leys, N.; Houdt, R.V.; Boon, N. Reevaluating multicolor flow cytometry to assess microbial viability. Appl. Microbiol. Biotechnol. 2016, 100, 9037–9051. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, L.; Chen, Y.; Long, Y. Optimization of staining with SYTO 9/propidium iodide: Interplay, kinetics and impact on Brevibacillus brevis. BioTechniques 2020, 69, 88–98. [Google Scholar] [CrossRef]
- Lee, J.; Yang, S.H.; Hong, S.-P.; Hong, D.; Lee, H.; Lee, H.-Y.; Kim, Y.-G.; Choi, I.S. Chemical control of yeast cell division by cross-linked shells of catechol-grafted polyelectrolyte multilayers. Macromol. Rapid Commun. 2013, 34, 1351–1356. [Google Scholar] [CrossRef]
- Ghani, R.; Mullish, B.H.; Roberts, L.A.; Davies, F.J.; Marchesi, J.R. The potential utility of fecal (or intestinal) microbiota transplantation in controlling infectious diseases. Gut Microbes 2022, 14, e2038856. [Google Scholar] [CrossRef]
- Cribby, S.; Taylor, M.; Reid, G. Vaginal microbiota and the use of probiotics. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 256490. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.Y.; Nguyen, D.T.; Kim, B.J.; Kim, N.; Kang, E.K.; Park, J.H.; Choi, I.S. Cytoprotection of Probiotic Lactobacillus acidophilus with Artificial Nanoshells of Nature-Derived Eggshell Membrane Hydrolysates and Coffee Melanoidins in Single-Cell Nanoencapsulation. Polymers 2023, 15, 1104. https://doi.org/10.3390/polym15051104
Han SY, Nguyen DT, Kim BJ, Kim N, Kang EK, Park JH, Choi IS. Cytoprotection of Probiotic Lactobacillus acidophilus with Artificial Nanoshells of Nature-Derived Eggshell Membrane Hydrolysates and Coffee Melanoidins in Single-Cell Nanoencapsulation. Polymers. 2023; 15(5):1104. https://doi.org/10.3390/polym15051104
Chicago/Turabian StyleHan, Sang Yeong, Duc Tai Nguyen, Beom Jin Kim, Nayoung Kim, Eunhye K. Kang, Ji Hun Park, and Insung S. Choi. 2023. "Cytoprotection of Probiotic Lactobacillus acidophilus with Artificial Nanoshells of Nature-Derived Eggshell Membrane Hydrolysates and Coffee Melanoidins in Single-Cell Nanoencapsulation" Polymers 15, no. 5: 1104. https://doi.org/10.3390/polym15051104
APA StyleHan, S. Y., Nguyen, D. T., Kim, B. J., Kim, N., Kang, E. K., Park, J. H., & Choi, I. S. (2023). Cytoprotection of Probiotic Lactobacillus acidophilus with Artificial Nanoshells of Nature-Derived Eggshell Membrane Hydrolysates and Coffee Melanoidins in Single-Cell Nanoencapsulation. Polymers, 15(5), 1104. https://doi.org/10.3390/polym15051104