Study of Candelilla Wax Concentrations on the Physical Properties of Edible Nanocoatings as a Function of Support Polysaccharides
Abstract
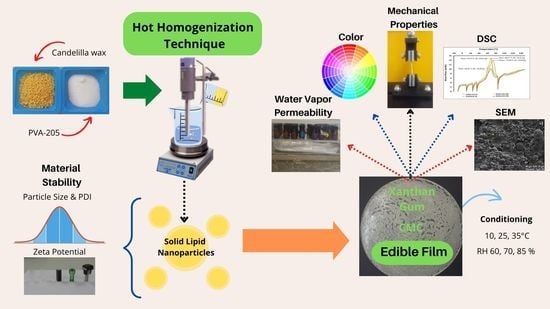
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solid Lipid Nanoparticle Preparation
2.3. Dynamic Light Scattering (DLS)
2.4. Film-Forming Dispersions
2.5. Film Formation
2.6. Water Vapor Permeability (WVP)
2.7. Mechanical Properties
2.8. Whiteness Index of Films
2.9. Differential Scanning Calorimetry (DSC)
2.10. Scanning Electron Microscopy (SEM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the SLN
3.2. Thickness and Water Vapor Permeability (WVP)
3.3. Mechanical Properties
3.4. Color
3.5. Differential Scanning Calorimetry (DSC)
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| ASTM | American Society for Testing and Materials |
| aw | Water activity |
| BSE+BSE (U) | backscattered electron (U shape) |
| CMC | carboxymethyl cellulose |
| DLS | dynamic light scattering |
| DSC | Differential Scanning Calorimeter |
| E | elongation at breaking |
| FDA | U.S. Food and Drug Administration |
| GRAS | generally recognized as safe |
| PDI | polydispersion index |
| PS | particle size |
| PVA | polyvinyl alcohol |
| RH | relative humidity |
| SEM | Scanning Electron Microscopy |
| SLN | solid lipid nanoparticles |
| Tmax | maximum temperature |
| TS | tensile strength |
| WI | whiteness index |
| WVP | water vapor permeability |
| WVT | water vapor transmission |
| XG | xanthan gum |
| YM | Young’s modulus |
| ΔE | total color difference |
| ΔH | enthalpy change |
| ζ | zeta potential |
References
- Aayush, K.; McClements, D.J.; Sharma, S.; Sharma, R.; Singh, G.P.; Sharma, K.; Oberoi, K. Innovations in the development and application of edible coatings for fresh and minimally processed Apple. Food Control 2022, 141, 109188. [Google Scholar] [CrossRef]
- Milani, J.M.; Nemati, A. Lipid-Based Edible Films and Coatings: A Review of Recent Advances and Applications. J. Packag. Technol. Res. 2022, 6, 11–22. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Tanner, C.; Cayot, P.; Karbowiak, T.; Debeaufort, F. Impact of functional properties and release kinetics on antioxidant activity of biopolymer active films and coatings. Food Chem. 2018, 242, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Shanmugam, M.; Bhat, R. Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Ehsani, A.; Kia, E.M.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Filho, J.G.D.O.; Albiero, B.R.; Calisto, H.; Bertolo, M.R.V.; Oldoni, F.C.A.; Egea, M.B.; Junior, S.B.; de Azeredo, H.M.C.; Ferreira, M.D. Bio-nanocomposite edible coatings based on arrowroot starch/cellulose nanocrystals/carnauba wax nanoemulsion containing essential oils to preserve quality and improve shelf life of strawberry. Int. J. Biol. Macromol. 2022, 219, 812–823. [Google Scholar] [CrossRef]
- García-Betanzos, C.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Del Real L, A.; Zambrano-zaragoza, M.L. The Evaluation of Mechanical, Thermal, Optical and Microstructural Properties of Edible Films with Solid Lipid Nanoparticles-Xanthan Gum Stored at Different Temperatures and Relative Humidities. Food Bioprocess Technol. 2016, 9, 1756–1768. [Google Scholar] [CrossRef]
- García-Betanzos, C.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Galindo-Pérez, M.; Zambrano-Zaragoza, M. Influence of solid lipid nanoparticle/xanthan gum coatings on compositional and enzymatic changes in guava (Psidium guajava L.) during ripening. Acta Hortic. 2018, 1194, 289–296. [Google Scholar] [CrossRef]
- Wiedenmann, V.; Oehlke, K.; Van Der Schaaf, U.; Koivula, H.M.; Mikkonen, K.S.; Karbstein, H.P. Emulsifier Composition of Solid Lipid Nanoparticles (SLN) Affects Mechanical and Barrier Properties of SLN-Protein Composite Films. J. Food Sci. 2019, 84, 3642–3652. [Google Scholar] [CrossRef]
- Yousuf, B.; Sun, Y.; Wu, S. Lipid and Lipid-containing Composite Edible Coatings and Films. Food Rev. Int. 2021, 38, 574–597. [Google Scholar] [CrossRef]
- Borges, A.; De Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gonzalez, M.F.S. Solid Lipid Nanoparticles and Applications. In Nanotechnology and Functional Foods; Sabliov, C., Chen, H., Yada, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 214–223. ISBN 9781118462201. [Google Scholar]
- Aranda-Ledesma, N.E.; Bautista-Hernández, I.; Rojas, R.; Aguilar-Zárate, P.; Medina-Herrera, N.d.P.; Castro-López, C.; Martínez-Ávila, G.C.G. Candelilla wax: Prospective suitable applications within the food field. LWT 2022, 159, 113170. [Google Scholar] [CrossRef]
- Núñez-García, I.C.; Rodríguez-Flores, L.G.; Guadiana-De-Dios, M.H.; González-Hernández, M.D.; Martínez-Ávila, G.C.G.; Gallegos-Infante, J.A.; González-Laredo, R.; Rosas-Flores, W.; Martínez-Gómez, V.J.; Rojas, R.; et al. Candelilla Wax Extracted by Traditional Method and an Ecofriendly Process: Assessment of Its Chemical, Structural and Thermal Properties. Molecules 2022, 27, 3735. [Google Scholar] [CrossRef] [PubMed]
- De León-Zapata, M.A.; Ventura-Sobrevilla, J.M.; Salinas-Jasso, T.A.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R.; Pastrana-Castro, L.; Rua-Rodríguez, M.L.; Aguilar, C.N. Changes of the shelf life of candelilla wax/tarbush bioactive based-nanocoated apples at industrial level conditions. Sci. Hortic. 2018, 231, 43–48. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Bernal-Couoh, T.F.; Quintanar-Guerrero, D.; de la Zambrano-Zaragoza, M.L. Physicochemical, total phenols and pectin methylesterase changes on quality maintenance on guava fruit ( Psidium guajava L.) coated with candeuba wax solid lipid nanoparticles-xanthan gum. Food Res. Int. 2017, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Totosaus, A.; Godoy, I.A.; Ariza-Ortega, T.J. Structural and mechanical properties of edible films from composite mixtures of starch, dextrin and different types of chemically modified starch. Int. J. Polym. Anal. Charact. 2020, 25, 517–528. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Rodrigues, R.; Vicente, A.A.; Pinheiro, A.C. Incorporation of Solid Lipid Nanoparticles into Stirred Yogurt: Effects in Physicochemical and Rheological Properties during Shelf-Life. Nanomaterials 2022, 13, 93. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Ardani, H.K.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. Enhancement of the Stability of Silver Nanoparticles Synthesized Using Aqueous Extract of Diospyros Discolor Willd. Leaves Using Polyvinyl Alcohol. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Chiyoda City, Japan, 2016; Volume 755, p. 11001. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.M.; Thunga, M.; Srinivasan, G.; Wang, S.; Kessler, M.R.; Grewell, D.; Yu, C.; Lamsal, B. Effect of TiO2 nanoparticles on thermo-mechanical properties of cast zein protein films. Food Packag. Shelf Life 2017, 13, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Syahida, S.N.; Ismail-Fitry, M.R.; Ainun, Z.M.A.; Hanani, Z.A.N. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life 2019, 23, 100437. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2015, 53, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, S.H.; Edwal, S.A.M.; Risyon, N.P.; Basha, R.K.; Talib, R.A. Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci. Technol. 2017, 37, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Maruddin, F.; Malaka, R.; Baba, S.; Amqam, H.; Taufik, M.; Sabil, S. Brightness, elongation and thickness of edible film with caseinate sodium using a type of plasticizer. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012043. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhatia, S.; Al-Azri, M.S.; Ullah, S.; Najmi, A.; Albratty, M.; Meraya, A.M.; Mohan, S.; Aldawsari, M.F. Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films. Membranes 2022, 12, 862. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2012, 115, 459–465. [Google Scholar] [CrossRef]
- Bosquez-Molina, E.; Guerrero-Legarreta, I.; Vernon-Carter, E. Moisture barrier properties and morphology of mesquite gum–candelilla wax based edible emulsion coatings. Food Res. Int. 2003, 36, 885–893. [Google Scholar] [CrossRef]
- Das, D.; Panesar, P.S.; Saini, C.S.; Kennedy, J.F. Improvement in properties of edible film through non-thermal treatments and nanocomposite materials: A review. Food Packag. Shelf Life 2022, 32, 100843. [Google Scholar] [CrossRef]
- Alvarez-Perez, O.B.; Ventura-Sobrevilla, J.M.; Torres-León, C.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Aguilar-González, M.A.; Aguilar, C.N. Development and characterization of whey protein films incorporated with tarbush polyphenols and candelilla wax. Food Biosci. 2021, 45, 101505. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Mauricio-Pérez, R.; González-Chávez, M.M.; Sánchez-Becerril, M.; Ornelas-Paz, J.D.J.; Pérez-Martínez, J.D. Physical properties of organogels and water in oil emulsions structured by mixtures of candelilla wax and monoglycerides. Food Res. Int. 2013, 54, 1360–1368. [Google Scholar] [CrossRef]
- Al-Hassan, A.; Norziah, M. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]



| Nomenclature | Component | |||
|---|---|---|---|---|
| SLN (g/L) | Glycerol (g/L) | XG (g/L) | CMC (g/L) | |
| SLN60, G10, XG | 60 | 10 | 3 | - |
| SLN20, G30, XG | 20 | 30 | 3 | - |
| SLN60, G30, CMC | 60 | 30 | - | 3 |
| SLN20, G10, CMC | 20 | 10 | - | 3 |
| Time (Week) | Particle Size (nm) | PDI | ζ (mV) |
|---|---|---|---|
| 0 | 809.7 ± 53.7 a | 0.08 ± 0.04 a | −18.8 ± 0.7 a |
| 1 | 829.6 ± 25.6 a | 0.10 ± 0.05 a | −14.2 ± 0.5 b |
| 2 | 866.4 ± 39.7 a | 0.12 ± 0.07 a | −8.7 ± 0.8 c |
| 3 | 879.6 ± 26.3 a | 0.17 ± 0.13 a,b | −7.9 ± 0.2 c |
| 4 | 882.8 ± 25.3 a | 0.24 ± 0.07 a,b | −5.7 ± 1.2 c,d |
| 5 | 885.9 ± 68.5 a | 0.31 ± 0.02 a,b | −3.5 ± 2.4 c,d |
| Thickness (mm) | WVP (g × mm/m2 × h × kPa) | Thickness (mm) | WVP (g × mm/m2 × h × kPa) | Thickness (mm) | WVP (g × mm/m2 × h × kPa) | |
|---|---|---|---|---|---|---|
| Formulation | 60% RH | 70% RH | 85% RH | |||
| 10 °C | ||||||
| SLN60, G10, XG | 0.119 ± 0.01 b,a | 0.483 ± 0.04 a,a | 0.101 ± 0.01 c,b | 0.418 ± 0.01 b,a | 0.097 ± 0.01 b,b | 0.166 ± 0.01 c,b |
| SLN20, G30, XG | 0.057 ± 0.01 b,b | 0.782 ± 0.01 a,b | 0.062 ± 0.01 b,a | 0.805 ± 0.01 a,a | 0.055 ± 0.01 c,b | 0.638 ± 0.01 a,c |
| SLN60, G30, CMC | 0.120 ± 0.01 b,b | 1.227 ± 0.11 a,b | 0.160 ± 0.01 a,a | 1.896 ± 0.01 a,a | 0.113 ± 0.01 c,b | 1.116 ± 0.01 a,b |
| SLN20, G10, CMC | 0.057 ± 0.00 b,b | 0.679 ± 0.02 a,c | 0.061 ± 0.01 a,a | 0.740 ± 0.01 a,b | 0.050 ± 0.01 b,c | 0.946 ± 0.03 a,a |
| 25 °C | ||||||
| SLN60, G10, XG | 0.122 ± 0.01 b,b | 0.134 ± 0.01 b,c | 0.131 ± 0.01 b,ab | 0.233 ± 0.01 c,b | 0.146 ± 0.02 a,a | 0.554 ± 0.01 a,a |
| SLN20, G30, XG | 0.058 ± 0.01 b,c | 0.209 ± 0.01 b,b | 0.064 ± 0.01 b,b | 0.342 ± 0.01 b,a | 0.071 ± 0.01 b,a | 0.361 ± 0.01 b,a |
| SLN60, G30, CMC | 0.137 ± 0.01 a,b | 0.581 ± 0.02 b,c | 0.143 ± 0.02 b,ab | 0.788 ± 0.03 b,b | 0.160 ± 0.01 b,a | 0.883 ± 0.02 b,a |
| SLN20, G10, CMC | 0.066 ± 0.01 a,b | 0.263 ± 0.06 b,b | 0.064 ± 0.00 a,b | 0.315 ± 0.01 b,ab | 0.075 ± 0.01 a,a | 0.379 ± 0.01 b,a |
| 35°C | ||||||
| SLN60, G10, XG | 0.137 ± 0.01 a,b | 0.096 ± 0.01 b,b | 0.150 ± 0.01 a,ab | 0.521 ± 0.02 a,a | 0.164 ± 0.02 a,a | 0.496 ± 0.01 b,a |
| SLN20, G30, XG | 0.063 ± 0.01 a,c | 0.208 ± 0.01 b,a | 0.068 ± 0.01 a,b | 0.212 ± 0.01 c,a | 0.081 ± 0.01 a,a | 0.203 ± 0.01 c,a |
| SLN60, G30, CMC | 0.142 ± 0.01 a,c | 0.443 ± 0.03 b,b | 0.151 ± 0.01 ab,b | 0.494 ± 0.01 c,b | 0.252 ± 0.01 a,a | 0.663 ± 0.03 c,a |
| SLN20, G10, CMC | 0.056 ± 0.01 b,c | 0.182 ± 0.01 b,b | 0.061 ± 0.01 a,b | 0.201 ± 0.01 c,ab | 0.077 ± 0.01 a,a | 0.211 ± 0.01 c,a |
| TS (MPa) | YM (MPa) | E (%) | TS (MPa) | YM (MPa) | E (%) | TS (MPa) | YM (MPa) | E (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Formulation | 60% RH | 70% RH | 85% RH | ||||||
| 10 °C | |||||||||
| SLN60, G10, XG | 8.01 ± 0.25 a,b | 5.72 ± 0.75 b,b | 4.03 ± 0.10 a,b | 7.58 ± 0.99 a,b | 6.73 ± 0.95 b,b | 3.71 ± 0.16 ab,b | 20.09 ± 1.49 a,a | 9.22 ± 0.27 a,a | 6.28 ± 0.31 a,a |
| SLN20, G30, XG | 1.17 ± 0.22 a,b | 0.02 ± 0.00 b,c | 59.12 ± 2.10 a,b | 0.75 ± 0.02 a,b | 0.03 ± 0.00 a,a | 82.07 ± 3.44 a,a | 1.80 ± 0.19 a,a | 0.03 ± 0.00 a,b | 53.21 ± 5.35 b,b |
| SLN60, G30, CMC | 2.28 ± 0.51 a,a | 1.13 ± 0.05 a,a | 17.00 ± 3.37 a,b | 2.04 ± 0.11 a,a | 1.12 ± 0.09 a,a | 24.39 ± 3.81 a,b | 2.47 ± 0.24 a,a | 0.60 ± 0.07 b,b | 49.05 ± 1.96 a,a |
| SLN20, G10, CMC | 2.11 ± 0.18 a,a | 0.30 ± 0.04 b,a | 24.49 ± 1.78 a,b | 1.64 ± 0.33 a,ab | 0.18 ± 0.00 ab,a | 24.64 ± 2.92 ab,b | 1.16 ± 0.12 b,b | 0.27 ± 0.06 b,a | 31.701 ± 1.57 a,a |
| 25 °C | |||||||||
| SLN60, G10, XG | 5.63 ± 0.72 b,a | 7.78 ± 0.92 b,a | 4.07 ± 0.37 a,ab | 5.07 ± 0.038 b,a | 6.57 ± 0.88 b,ab | 3.37 ± 0.63 b,b | 3.56 ± 0.20 b,b | 4.67 ± 0.35 c,b | 5.11 ± 0.16 b,a |
| SLN20, G30, XG | 0.41 ± 0.01 b,a | 0.02 ± 0.00 b,a | 39.50 ± 7.79 b,a | 0.34 ± 0.00 b,b | 0.01 ± 0.00 c,b | 44.96 ± 6.94 b,a | 0.33 ± 0.01 b,b | 0.01 ± 0.00 b,b | 51.36 ± 1.89 b,a |
| SLN60, G30, CMC | 1.95 ± 0.05 a,a | 1.52 ± 0.19 a,a | 14.29 ± 0.91 a,b | 1.28 ± 0.24 b,b | 0.65 ± 0.20 b,b | 20.87 ± 1.12 a,a | 1.50 ± 0.07 b,b | 1.01 ± 0.07 ab,b | 19.89 ± 0.88 b,a |
| SLN20, G10, CMC | 2.71 ± 0.19 ab,a | 0.43 ± 0.01 ab,a | 26.10 ± 1.37 a,a | 1.69 ± 0.09 a,b | 0.22 ± 0.01 a,b | 22.17 ± 3.05 b,a | 1.42 ± 0.38 ab,b | 0.21 ± 0.01 b,b | 25.44 ± 1.44 b,a |
| 35 °C | |||||||||
| SLN60, G10, XG | 5.41 ± 0.46 b,a | 10.56 ± 0.77 a,a | 3.47 ± 0.30 a,a | 5.73 ± 0.69 b,a | 11.39 ± 1.58 a,a | 4.64 ± 0.55 a,a | 5.13 ± 1.22 b,a | 7.26 ± 0.16 b,b | 4.17 ± 0.54 c,a |
| SLN20, G30, XG | 1.13 ± 0.02 a,a | 0.054 ± 0.01 a,a | 67.15 ± 8.39 a,b | 0.64 ± 0.05 c,b | 0.028 ± 0.00 b,b | 48.37 ± 2.69 b,c | 0.61 ± 0.06 b,b | 0.03 ± 0.00 a,b | 93.23 ± 5.00 a,a |
| SLN60, G30, CMC | 1.72 ± 0.53 a,a | 1.41 ± 0.22 a,a | 14.31 ± 2.79 a,b | 0.37 ± 0.02 c,b | 0.026 ± 0.00 c,b | 100.68 ± 15.07 b,a | 1.13 ± 0.16 b,ab | 1.26 ± 0.34 a,a | 13.62 ± 0.86 a,b |
| SLN20, G10, CMC | 2.76 ± 0.32 b,a | 0.53 ± 0.08 a,a | 26.27 ± 2.80 a,b | 1.77 ± 0.05 a,b | 0.12 ± 0.04 b,b | 30.93 ± 2.18 a,ab | 1.97 ± 0.29 a,b | 0.53 ± 0.01 a,a | 34.54 ± 3.38 a,a |
| ΔE | WI | ΔE | WI | ΔE | WI | |
|---|---|---|---|---|---|---|
| Formulation | 60% RH | 70% RH | 85% RH | |||
| 10 °C | ||||||
| SLN60, G10, XG | 4.43 ± 1.06 b,a | 96.38 ± 0.28 a,a | 4.67 ± 0.65 b,a | 96.33 ± 0.23 a,a | 4.89 ± 0.36 b,a | 96.10 ± 0.37 a,a |
| SLN20, G30, XG | 3.34 ± 0.20 b,b | 95.65 ± 0.37 ab,a | 3.46 ± 0.11 b,b | 95.70 ± 0.43 a,a | 4.53 ± 0.06 a,a | 96.26 ± 0.09 a,a |
| SLN60, G30, CMC | 3.94 ± 0.34 b,b | 95.03 ± 0.17 a,b | 3.63 ± 0.33 b,b | 95.00 ± 0.18 a,b | 5.16 ± 0.18 c,a | 95.99 ± 0.19 a,a |
| SLN20, G10, CMC | 3.60 ± 0.29 b,a | 96.45 ± 0.56 a,a | 4.08 ± 0.58 a,a | 95.87 ± 0.08 a,a | 4.00 ± 0.67 a,a | 95.93 ± 0.18 a,a |
| 25°C | ||||||
| SLN60, G10, XG | 6.21 ± 1.01 ab,a | 95.18 ± 0.12 b,a | 5.52 ± 0.73 b,a | 94.17 ± 0.54 b,ab | 5.84 ± 0.96 b,a | 93.71 ± 0.71 b,b |
| SLN20, G30, XG | 3.48 ± 0.27 b,b | 96.19 ± 0.36 a,a | 4.08 ± 0.76 ab,ab | 95.65 ± 0.46 a,a | 4.96 ± 0.63 a,a | 95.32 ± 0.41 b,a |
| SLN60, G30, CMC | 5.63 ± 0.13 a,b | 95.45 ± 0.16 a,a | 6.61 ± 0.95 a,ab | 95.10 ± 0.68 a,a | 7.93 ± 0.40 a,a | 94.49 ± 0.19 b,a |
| SLN20, G10, CMC | 4.01 ± 0.15 ab,a | 96.27 ± 0.31 a,a | 5.08 ± 0.76 a,a | 95.26 ± 0.77 a,ab | 4.86 ± 0.10 a,a | 94.82 ± 0.45 b,b |
| 35°C | ||||||
| SLN60, G10, XG | 7.33 ± 0.36 a,a | 94.16 ± 0.39 c,a | 6.59 ± 0.33 a,b | 91.28 ± 1.12 c,b | 6.96 ± 0.13 a,ab | 92.72 ± 0.75 b,ab |
| SLN20, G30, XG | 4.82 ± 0.79 a,a | 94.76 ± 0.77 b,a | 5.26 ± 0.58 a,a | 94.82 ± 0.11 a,a | 5.04 ± 0.47 a,a | 94.79 ± 0.37 b,a |
| SLN60, G30, CMC | 6.28 ± 0.84 a,a | 93.70 ± 0.53 b,a | 6.61 ± 0.31 a,a | 93.90 ± 0.76 a,a | 6.44 ± 0.53 b,a | 93.80 ± 0.44 b,a |
| SLN20, G10, CMC | 4.92 ± 0.71 a,a | 95.34 ± 0.56 a,a | 4.89 ± 0.37 a,a | 93.76 ± 0.62 b,b | 4.90 ± 0.54 a,a | 94.55 ± 0.03 b,ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Betanzos, C.I.; Hernández-Sánchez, H.; Ojeda-Piedra, S.A.; Ulloa-Saavedra, A.; Quintanar-Guerrero, D.; Zambrano-Zaragoza, M.L. Study of Candelilla Wax Concentrations on the Physical Properties of Edible Nanocoatings as a Function of Support Polysaccharides. Polymers 2023, 15, 1209. https://doi.org/10.3390/polym15051209
García-Betanzos CI, Hernández-Sánchez H, Ojeda-Piedra SA, Ulloa-Saavedra A, Quintanar-Guerrero D, Zambrano-Zaragoza ML. Study of Candelilla Wax Concentrations on the Physical Properties of Edible Nanocoatings as a Function of Support Polysaccharides. Polymers. 2023; 15(5):1209. https://doi.org/10.3390/polym15051209
Chicago/Turabian StyleGarcía-Betanzos, Claudia I., Humberto Hernández-Sánchez, Sergio A. Ojeda-Piedra, Araceli Ulloa-Saavedra, David Quintanar-Guerrero, and María L. Zambrano-Zaragoza. 2023. "Study of Candelilla Wax Concentrations on the Physical Properties of Edible Nanocoatings as a Function of Support Polysaccharides" Polymers 15, no. 5: 1209. https://doi.org/10.3390/polym15051209
APA StyleGarcía-Betanzos, C. I., Hernández-Sánchez, H., Ojeda-Piedra, S. A., Ulloa-Saavedra, A., Quintanar-Guerrero, D., & Zambrano-Zaragoza, M. L. (2023). Study of Candelilla Wax Concentrations on the Physical Properties of Edible Nanocoatings as a Function of Support Polysaccharides. Polymers, 15(5), 1209. https://doi.org/10.3390/polym15051209