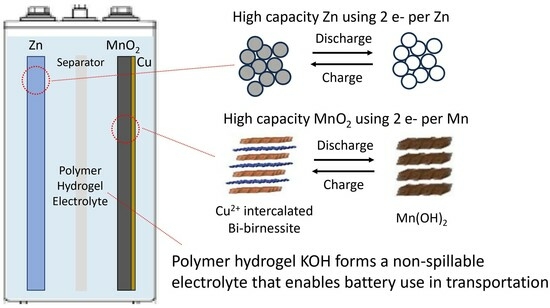
Use of Hydrogel Electrolyte in Zn-MnO2 Rechargeable Batteries: Characterization of Safety, Performance, and Cu2+ Ion Diffusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Synthesis
2.2. Battery Preparation
2.3. Electrolyte Spillability Safety Measurements
2.4. Electrochemical Measurements
2.5. Cu Diffusion Coefficient
3. Results and Discussion
3.1. Non-Spillable Hydrogel Experiment
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallaway, J.W.; Erdonmez, C.K.; Zhong, Z.; Croft, M.; Sviridov, L.A.; Sholklapper, T.Z.; Turney, D.E.; Banerjee, S.; Steingart, D.A. Real-time materials evolution visualized within intact cycling alkaline batteries. J. Mater. Chem. A 2014, 2, 2757–2764. [Google Scholar] [CrossRef]
- Turney, D.E.; Gallaway, J.W.; Yadav, G.G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Weigart, Y.K.; Wang, J.; D’Ambrose, M.J.; Kolhekar, S.; et al. Rechargeable zinc alkaline anodes for long-cycle energy storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Ingale, N.D.; Gallaway, J.W.; Nyce, M.; Couzis, A.; Banerjee, S. Rechargeability and economic aspects of alkaline zinc-manganese dioxide cells for electrical storage and load leveling. J. Power Sources 2015, 276, 7–18. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N.; Chalamala, B.R. Rechargeable alkaline zinc-manganese oxide batteries for grid storage: Mechanisms, challenges and developments. Mater. Sci. Eng. 2021, 143, 100593. [Google Scholar] [CrossRef]
- D’Ambrose, M.J.; Turney, D.E.; Yadav, G.G.; Nyce, M.; Banerjee, S. Material failure mechanisms of alkaline Zn rechargeable conversion electrodes. ACS Appl. Energy Mater. 2021, 4, 3381–3392. [Google Scholar] [CrossRef]
- Hawkins, B.E.; Turney, D.E.; Messinger, R.J.; Kiss, A.M.; Yadav, G.G.; Banerjee, S.; Lambert, T.N. Electroactive ZnO: Mechanisms, Conductivity, and Advances in Zn Alkaline Battery Cycling. Adv. Energy Mater. 2022, 12, 2103294. [Google Scholar] [CrossRef]
- Gallaway, J.W.; Yadav, G.G.; Turney, D.E.; Nyce, M.; Huang, J.; Chen-Weigart, Y.-C.K.; Williams, G.; Thieme, J.; Okasinksi, J.S.; Wei, X. An operando study of the initial discharge of Bi and Bi/Cu modified MnO2. J. Electrochem. Soc. 2018, 165, A2935. [Google Scholar] [CrossRef]
- Seo, J.K.; Shin, J.; Chung, H.; Meng, P.Y.; Wang, X.; Meng, Y.S. Intercalation and Conversion Reactions of Nanosized β-MnO2 Cathode in the Secondary Zn/MnO2 Alkaline Battery. J. Phys. Chem. C 2018, 122, 11177–11185. [Google Scholar] [CrossRef]
- Yadav, G.G.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Huang, J.; Wei, X.; Banerjee, S. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries. Nat. Commun. 2017, 8, 14424. [Google Scholar] [CrossRef]
- Yadav, G.G.; Wei, X.; Huang, J.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Secor, J.; Banerjee, S. A conversion-based highly energy dense Cu2+ intercalated Bi-birnessite/Zn alkaline battery. J. Mater. Chem. A 2017, 5, 15845–15854. [Google Scholar] [CrossRef]
- Bruck, A.M.; Kim, M.A.; Ma, L.; Ehrlich, S.N.; Okasinksi, J.S.; Gallaway, J.W. Bismuth enables the formation of disordered birnessite in rechargeable alkaline batteries. J. Electrochem. Soc. 2020, 167, 110514. [Google Scholar] [CrossRef]
- Schorr, N.B.; Arnot, D.J.; Bruck, A.M.; Duay, J.; Kelly, M.; Having, R.L.; Ricketts, L.S.; Vigil, J.A.; Gallaway, J.W.; Lambert, T.N. Rechargeable alkaline Zinc/Copper oxide batteries. ACS Appl. Energy Mater. 2021, 4, 7073–7082. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, S.; Wu, S.; Ma, X.; Hussain, I.; Sun, Z.; Lu, Z.; Zhang, K. Copper activated near-full two-electron Mn4+/Mn2+ redox for mild aqueous Zn/MnO2 battery. Chem. Eng. J. 2022, 450, 137923. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, S.; Ren, Y.; Jiang, S.; Pan, X.; Sun, G.; Zhu, B.; Wen, Y.; Li, X.; Tu, F.; et al. Promoting cycle stability and rate performance of birnessite-type MnO2 cathode via cupper and bismuth dual ions pre-intercalation for aqueous zinc-ion batteries. J. Energy Storage 2023, 74, 109589. [Google Scholar] [CrossRef]
- Cho, J.; Yadav, G.G.; Weiner, M.; Huang, J.; Upreti, A.; Wei, X.; Yakobov, R.; Hawkins, B.E.; Nyce, M.; Lambert, T.N.; et al. Hydroxyl conducting hydrogels enable low-maintenance commercially sized rechargeable Zn–MnO2 Batteries for Use in Solar Microgrids. Polymers 2022, 14, 417. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, H.; Cao, Y.; Ai, X. Preparation and electrochemical characterization of the alkaline polymer gel electrolyte polymerized from acrylic acid and KOH solution. Electrochim. Acta 2004, 49, 2533–2539. [Google Scholar] [CrossRef]
- Mohamad, A.A. Zn/gelled 6M KOH/O2 zinc-air battery. J. Power Sources 2006, 159, 752–757. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Sampath, S.; Shukla, A.K. Hydrogel-polymer electrolytes for electrochemical capacitors: An overview. Energy Environ. Sci. 2009, 2, 55–67. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Li, S.; Fan, X.; Liu, X.; Zhao, Z.; Xu, W.; Wu, Z.; Feng, Z.; Zhong, C.; Hu, W. Potassium Polyacrylate-Based Gel Polymer Electrolyte for Practical Zn−Ni Batteries. ACS Appl. Mater. Interfaces 2022, 14, 22847–22857. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Li, M.; Gao, G.; Fan, H.; Ma, L. The Gel-State Electrolytes in Zinc-Ion Batteries. Batteries 2022, 8, 214. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, Z.; Gao, C.; Jin, S.; Zhang, S.; Wang, X.; Zhou, H. Polyacrylic acid assisted synthesis of free-standing MnO2/CNTs cathode for Zinc-ion batteries. Nanotechnology 2020, 31, 375–401. [Google Scholar] [CrossRef] [PubMed]
- The Code of Federal Regulations (CFR), 49 CFR 173. Available online: https://www.ecfr.gov/current/title-49/subtitle-B/chapter-I/subchapter-C/part-173/subpart-E/section-173.159a?msclkid=7e67d3fdb43911ecb601bcd46a0d0bb0 (accessed on 31 December 2023).
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical impedance spectroscopy for all-solid-state batteries: Theory, methods and future outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Sun, M.; Ji, G.; Zheng, J. A hydrogel electrolyte with ultrahigh ionic conductivity and transference number benefit from Zn2+ “highways” for dendrite-free Zn-MnO2 battery. Chem. Eng. J. 2023, 463, 142535. [Google Scholar] [CrossRef]
- Tél, A.; Bauer, R.A.; Varga, Z.; Zrínyi, M. Heat conduction in poly(N-isopropylacrylamide) hydrogels. Int. J. Therm. Sci. 2014, 85, 47–53. [Google Scholar] [CrossRef]
- Han, J.; Jang, S.; Kim, B.-K.; Park, K. Electrochemical study of agarose hydrogels for natural convection on macroelectrodes and ultramicroelectrodes. J. Anal. Sci. Technol. 2023, 14, 10. [Google Scholar] [CrossRef]






| Mole Fraction MBA:H2O | Flow from ~1 mm Gap | Flow from ~75 mm Gap |
|---|---|---|
| 2.61 × 10−5 | Flow | Flow |
| 3.40 × 10−5 | Flow | Flow |
| 3.92 × 10−5 | No Flow | Flow |
| 4.70 × 10−5 | No Flow | Flow |
| 5.20 × 10−5 | No Flow | Flow |
| 6.00 × 10−5 | No Flow | Flow |
| 6.50 × 10−5 | No Flow | No Flow |
| 7.30 × 10−5 | No Flow | No Flow |
| 7.80 × 10−5 | No Flow | No Flow |
| 1st Cycle | 5th Cycle | 12th Cycle | |
|---|---|---|---|
| Liquid KOH Electrolyte |  |  |  |
| Gel Electrolyte |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.; Turney, D.E.; Yadav, G.G.; Nyce, M.; Wygant, B.R.; Lambert, T.N.; Banerjee, S. Use of Hydrogel Electrolyte in Zn-MnO2 Rechargeable Batteries: Characterization of Safety, Performance, and Cu2+ Ion Diffusion. Polymers 2024, 16, 658. https://doi.org/10.3390/polym16050658
Cho J, Turney DE, Yadav GG, Nyce M, Wygant BR, Lambert TN, Banerjee S. Use of Hydrogel Electrolyte in Zn-MnO2 Rechargeable Batteries: Characterization of Safety, Performance, and Cu2+ Ion Diffusion. Polymers. 2024; 16(5):658. https://doi.org/10.3390/polym16050658
Chicago/Turabian StyleCho, Jungsang, Damon E. Turney, Gautam Ganapati Yadav, Michael Nyce, Bryan R. Wygant, Timothy N. Lambert, and Sanjoy Banerjee. 2024. "Use of Hydrogel Electrolyte in Zn-MnO2 Rechargeable Batteries: Characterization of Safety, Performance, and Cu2+ Ion Diffusion" Polymers 16, no. 5: 658. https://doi.org/10.3390/polym16050658
APA StyleCho, J., Turney, D. E., Yadav, G. G., Nyce, M., Wygant, B. R., Lambert, T. N., & Banerjee, S. (2024). Use of Hydrogel Electrolyte in Zn-MnO2 Rechargeable Batteries: Characterization of Safety, Performance, and Cu2+ Ion Diffusion. Polymers, 16(5), 658. https://doi.org/10.3390/polym16050658