Keratin/Polyvinyl Alcohol Blend Films Cross-Linked by Dialdehyde Starch and Their Potential Application for Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
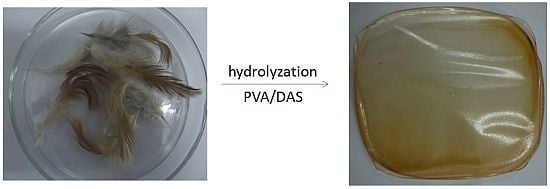
2.2. Preparation of the Blended Films and the Dye-Loaded Films
2.3. Characterization
2.4. Dye Release
3. Results and Discussion
3.1. Morphology of the Films

3.2. X-Ray Diffraction (XRD) Analysis

3.3. DSC Analysis

| Sample | Te (°C) | ∆He (J·K−1) | Tm (°C) | ∆Hm (J·K−1) |
|---|---|---|---|---|
| FK | 138.5 | 232.0 | 214.8 | 267.5 |
| P-30 | 132.5 | 303.7 | 217.2 | 314.2 |
| P-30-15 | 143.2 | 149.7 | 216.0 | 150.5 |
| PVA | 131.4 | 296.1 | 225.8 | 461.2 |
3.4. TSM of the Blend Films

3.5. Rhodamine B Release from the PVA/FK Film

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rocha Plácido Moore, G.; Maria Martelli, S.; Gandolfo, C.; José do Amaral Sobral, P.; Borges Laurindo, J. Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocoll. 2006, 20, 975–982. [Google Scholar]
- Kundu, B.; Kurland, N.E.; Yadavalli, V.K.; Kundu, S.C. Isolation and processing of silk proteins for biomedical applications. Int. J. Biol. Macromol. 2014, 70, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-B.; Jo, W.-S.; Song, H.-Y.; Chung, K.-S.; Won, M.; Song, K.B. Effects of plasticizers and nano-clay content on the physical properties of chicken feather protein composite films. Food Hydrocoll. 2013, 31, 340–345. [Google Scholar] [CrossRef]
- Verbeek, C.J.R.; van den Berg, L.E. Recent developments in thermo-mechanical processing of proteinous bioplastics. Recent Pat. Mater. Sci. 2009, 2, 171–189. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.; Sharif, A.; Kalaee, M.; Nouri, A.; Manafi, M. Morphology, nanomechanical and thermodynamic surface characteristics of nylon 6/feather keratin blend films: An atomic force microscopy investigation. Polym. Int. 2012, 61, 646–656. [Google Scholar] [CrossRef]
- Flores-Hernández, C.; Colín-Cruz, A.; Velasco-Santos, C.; Castaño, V.; Rivera-Armenta, J.; Almendarez-Camarillo, A.; García-Casillas, P.; Martínez-Hernández, A. All green composites from fully renewable biopolymers: Chitosan-starch reinforced with keratin from feathers. Polymers 2014, 6, 686–705. [Google Scholar] [CrossRef]
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Vineis, C.; Ceria, A.; Tonin, C. Composite biomaterials from fibre wastes: Characterization of wool-cellulose acetate blends. Compos. A 2008, 39, 126–132. [Google Scholar] [CrossRef]
- Martelli, S.M.; Moore, G.R.P.; Laurindo, J.B. Mechanical properties, water vapor permeability and water affinity of feather keratin films plasticized with sorbitol. J. Polym. Environ. 2006, 14, 215–222. [Google Scholar] [CrossRef]
- Barone, J.R.; Schmidt, W.F. Nonfood applications of proteinaceous renewable materials. J. Chem. Educ. 2006, 83, 1003–1009. [Google Scholar] [CrossRef]
- Prasong, S.; Wasan, T. Preparation and characterization of hair keratin/gelatin blend films. Pak. J. Biol. Sci. 2011, 14, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Okitsu, N.; Tachibana, A.; Yamauchi, K. Preparation and characterization of keratin-chitosan composite film. Biomaterials 2002, 23, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Tian-Yu, K.; Dong-Xia, L.; Xu-Hong, Y. Preparation and properties of keratin/cmc blend membranes. Adv. Mater. Res. 2013, 647, 190–194. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, A.; Boopalan, K.; Radhakrishnan, G.; Sankaran, S.; Das, B.N.; Sastry, T.P. Preparation of feather keratin hydrolyzate-gelatin composites and their graft copolymers. J. Macromol. Sci. A. 2005, 42, 1703–1713. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Patrucco, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Subramanian, U.M.; Kumar, S.V.; Nagiah, N.; Sivagnanam, U.T. Fabrication of polyvinyl alcohol-polyvinylpyrrolidone blend scaffolds via electrospinning for tissue engineering applications. Int. J. Polym. Mater. 2014, 63, 462–470. [Google Scholar] [CrossRef]
- Mao, Y.; Mohanty, P.; Ghosh, G. Biomimetic apatite-coated porous pva scaffolds promote the growth of breast cancer cells. Mater. Sci. Eng. C 2014, 44, 310–316. [Google Scholar] [CrossRef]
- Alves, P.M.A.; Carvalho, R.A.; Moraes, I.C.F.; Luciano, C.G.; Bittante, A.M.Q.B.; Sobral, P.J.A. Development of films based on blends of gelatin and poly(vinyl alcohol) cross linked with glutaraldehyde. Food Hydrocoll. 2011, 25, 1751–1757. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Properties of biodegradable blend films based on fish myofibrillar protein and polyvinyl alcohol as influenced by blend composition and ph level. J. Food Eng. 2010, 100, 85–92. [Google Scholar] [CrossRef]
- Su, J.-F.; Yuan, X.-Y.; Huang, Z.; Xia, W.-L. Properties stability and biodegradation behaviors of soy protein isolate/poly (vinyl alcohol) blend films. Polym. Degrad. Stab. 2010, 95, 1226–1237. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/PVA blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, B.-S.; Shin, H.K.; Park, S.-J.; Kim, H.-Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C 2013, 33, 5051–5057. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Weller, C.L.; Cezeirat, C.; Hanna, M.A. Soy protein isolate dialdehyde starch films. Ind. Crop. Prod. 1998, 8, 195–203. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Handa, A.; Weller, C.L.; Hanna, M.A. Solubility, tensile, and color properties of modified soy protein isolate films. J. Agric. Food Chem. 2000, 48, 4937–4941. [Google Scholar] [CrossRef] [PubMed]
- Langmaler, F.; Mokrejs, P.; Kolomamik, K.; Mladek, M. Biodegradable packing materials from hydrolysates of collagen waste proteins. Waste Manag. 2008, 28, 549–556. [Google Scholar] [CrossRef]
- Parris, N.; Coffin, D.R. Composition factors affecting the water vapor permeability and tensile properties of hydrophilic zein films. J. Agric. Food Chem. 1997, 45, 1596–1599. [Google Scholar]
- Mokrejs, P.; Langmaier, F.; Janacova, D.; Mladek, M.; Kolomaznik, K.; Vasek, V. Thermal study and solubility tests of films based on amaranth flour starch-protein hydrolysate. J. Therm. Anal. Calorim. 2009, 98, 299–307. [Google Scholar]
- Ustunol, Z.; Mert, B. Water solubility, mechanical, barrier, and thermal properties of cross-linked whey protein isolate-based films. J. Food Sci. 2004, 69, 129–133. [Google Scholar]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and physicochemical properties of dialdehyde starch crosslinked feather keratin/pva composite films. J. Macromol. Sci. A 2014, 51, 1009–1015. [Google Scholar] [CrossRef]
- Ramos, O.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Fatima Pocas, M.; Pintado, M.E.; Xavier Malcata, F. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, M.; Freddi, G.; Crighton, J.S. Structure and compatibility of poly(vinyl alcohol)-silk fibroin (PVA/SA) blend films. J. Polym. Sci. B Polym. Phys. 1994, 32, 243–248. [Google Scholar] [CrossRef]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Nakano, Y.; Bin, Y.; Bando, M.; Nakashima, T.; Okuno, T.; Kurosu, H.; Matsuo, M. Structure and mechanical properties of chitosan/poly(vinyl alcohol) blend films. Macromol. Symp. 2007, 258, 63–81. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Effect of crosslinking on the properties of sodium caseinate films. J. Appl. Polym. Sci. 2010, 116, 18–26. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Villalobos, R.; Chiralt, A. Effect of cross-linking using aldehydes on properties of glutenin-rich films. Food Hydrocoll. 2004, 18, 403–411. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile properties, barrier properties, and biodegradation in soil of compression-molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Elizondo, N.J.; Sobral, P.J.A.; Menegalli, F.C. Development of films based on blends of amaranthus cruentus flour and poly(vinyl alcohol). Carbohydr. Polym. 2009, 75, 592–598. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y.; Savina, I.N. Keratin/Polyvinyl Alcohol Blend Films Cross-Linked by Dialdehyde Starch and Their Potential Application for Drug Release. Polymers 2015, 7, 580-591. https://doi.org/10.3390/polym7030580
Dou Y, Zhang B, He M, Yin G, Cui Y, Savina IN. Keratin/Polyvinyl Alcohol Blend Films Cross-Linked by Dialdehyde Starch and Their Potential Application for Drug Release. Polymers. 2015; 7(3):580-591. https://doi.org/10.3390/polym7030580
Chicago/Turabian StyleDou, Yao, Buning Zhang, Ming He, Guoqiang Yin, Yingde Cui, and Irina N. Savina. 2015. "Keratin/Polyvinyl Alcohol Blend Films Cross-Linked by Dialdehyde Starch and Their Potential Application for Drug Release" Polymers 7, no. 3: 580-591. https://doi.org/10.3390/polym7030580
APA StyleDou, Y., Zhang, B., He, M., Yin, G., Cui, Y., & Savina, I. N. (2015). Keratin/Polyvinyl Alcohol Blend Films Cross-Linked by Dialdehyde Starch and Their Potential Application for Drug Release. Polymers, 7(3), 580-591. https://doi.org/10.3390/polym7030580