The Impact of Polyvinylpyrrolidone on Properties of Cadmium Oxide Semiconductor Nanoparticles Manufactured by Heat Treatment Technique
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Synthesis of the Nanoparticles
2.3. Characterization
3. Results and Discussion
3.1. Effect of Calcinations Temperature on Structural, Morphology, Phase Composition, Optical Properties
3.2. Impact of PVP Concentration on Structure, Morphology, Phase Composition, Optical, and Magnetic Properties
3.2.1. Structural Characterization
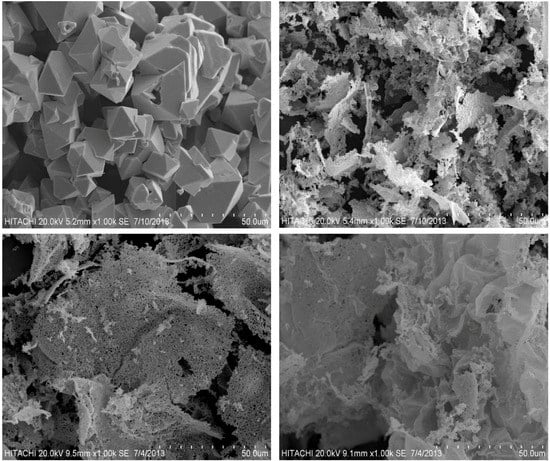
3.2.2. Surface Morphology
3.2.3. TEM Study
3.2.4. Phase Composition
3.2.5. UV–Vis Study
3.2.6. Photoluminescence Study
3.2.7. Electron Spin Resonance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Hau, S.K.; Yip, H.-L.; Baek, N.S.; Zou, J.; O’Malley, K.; Jen, A.K.-Y. Air-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layer. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Bouclé, J.; Ravirajan, P.; Nelson, J. Hybrid polymer-metal oxide thin films for photovoltaic applications. J. Mater. Chem. 2007, 17, 3141–3153. [Google Scholar] [CrossRef]
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 2004, 34, 151–180. [Google Scholar] [CrossRef]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.K.; Chandra, U.; Sherigara, B.; Jayadevappa, H. Synthesis of cdo nanoparticles and their modified carbon paste electrode for determination of dopamine and ascorbic acid by using cyclic voltammetry technique. Int. J. Electrochem. Sci. 2010, 5, 10–17. [Google Scholar]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanopart. Res. 2012, 14, 1–24. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles—Properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Porel, S.; Radhakrishnan, T. Polymer thin films embedded with in situ grown metal nanoparticles. Chem. Soc. Rev. 2009, 38, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, S.; Bronstein, L.; Davankov, V.; Tsyurupa, M.; Solodovnikov, S.; Valetsky, P.; Wilder, E.; Spontak, R. Cobalt nanoparticle formation in the pores of hyper-cross-linked polystyrene: Control of nanoparticle growth and morphology. Chem. Mater. 1999, 11, 3210–3215. [Google Scholar] [CrossRef]
- Rozenberg, B.; Tenne, R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization—Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, A. A facile thermal-treatment route to synthesize the semiconductor CdO nanoparticles and effect of calcination. Mater. Sci. Semicond. Process. 2014, 26, 460–466. [Google Scholar] [CrossRef]
- Antonietti, M.; Göltner, C. Superstructures of functional colloids: Chemistry on the nanometer scale. Angew. Chem. Int. Ed. Engl. 1997, 36, 910–928. [Google Scholar] [CrossRef]
- Schmid, G.; Chi, L.F. Metal clusters and colloids. Adv. Mater. 1998, 10, 515–526. [Google Scholar] [CrossRef]
- Reetz, M.T.; Helbig, W. Size-selective synthesis of nanostructured transition metal clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Fendler, J.H. Nanoparticles and Nanostructured Films: Preparation, Characterization, and Applications; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Bigg, D. Thermal conductivity of heterophase polymer compositions. In Thermal and Electrical Conductivity of Polymer Materials; Springer: New York, NY, USA, 1995; pp. 1–30. [Google Scholar]
- Park, J.; Lee, E.; Hwang, N.M.; Kang, M.; Kim, S.C.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew. Chem. 2005, 117, 2932–2937. [Google Scholar] [CrossRef]
- Noguchi, T.; Gotoh, K.; Yamaguchi, Y.; Deki, S. Novel method to disperse ultrafine metal particles into polymer. J. Mater. Sci. Lett. 1991, 10, 477–479. [Google Scholar] [CrossRef]
- Spatz, J.P.; Herzog, T.; Mößmer, S.; Ziemann, P.; Möller, M. Micellar inorganic-polymer hybrid systems—A tool for nanolithography. Adv. Mater. 1999, 11, 149–153. [Google Scholar] [CrossRef]
- Selvan, T.; Spatz, J.P.; Klok, H.-A.; Möller, M. Gold-polypyrrole core-shell particles in diblock copolymer micelles. Adv. Mater. 1998, 10, 132–134. [Google Scholar] [CrossRef]
- Spatz, J.P.; Mössmer, S.; Hartmann, C.; Möller, M.; Herzog, T.; Krieger, M.; Boyen, H.-G.; Ziemann, P.; Kabius, B. Ordered deposition of inorganic clusters from micellar block copolymer films. Langmuir 2000, 16, 407–415. [Google Scholar] [CrossRef]
- Kumar, R.V.; Koltypin, Y.; Palchik, O.; Gedanken, A. Preparation and characterization of nickel-polystyrene nanocomposite by ultrasound irradiation. J. Appl. Polym. Sci. 2002, 86, 160–165. [Google Scholar] [CrossRef]
- Lei, Z.; Wei, X.; Fan, Y.; Liu, Y.; Bi, S. Fabrication of CdSe composite by using the amphiphilic block copolymer as template. J. Colloid Interface Sci. 2006, 304, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-J.; Wang, H. Synthesis of metal chalcogenide nanoparticles. In Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 10, pp. 347–367. [Google Scholar]
- PEI, L.W. Architectural Control of Metal Sulfide Nanocrystals and Polymer Composites. Ph.D. Thesis, National University of Singapore, Singapore, 2006. [Google Scholar]
- Liu, T.; Burger, C.; Chu, B. Nanofabrication in polymer matrices. Prog. Polym. Sci. 2003, 28, 5–26. [Google Scholar] [CrossRef]
- Ortega, M.; Santana, G.; Morales-Acevedo, A. Optoelectronic properties of CdO-Si heterojunctions. Superf. Vaico 1999, 9, 294–295. [Google Scholar]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Sravani, C.; Reddy, K.; Hussain, O.M.; Reddy, P.J. Investigations on n-CdO/p-CdTe thin film heterojunctions. Thin Solid Films 1994, 253, 339–343. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, M.; Liu, F.; Chen, G.; Xu, L.; Kong, X. Overview on the synthesis and applications of cadmium hydroxide nanomaterials. J. Iran Chem. Soc. 2015. [Google Scholar] [CrossRef]
- Zaien, M.; Ahmed, N.; Hassan, Z. Fabrication and characterization of nanocrystalline n-CdO/p-Si as a solar cell. Superlattice Microstruct. 2012, 52, 800–806. [Google Scholar] [CrossRef]
- Hameş, Y.; San, S.E. Cdo/Cu2O solar cells by chemical deposition. Sol. Energy 2004, 77, 291–294. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Liu, J. Highly porous CdO nanowires: Preparation based on hydroxy-and carbonate-containing cadmium compound precursor nanowires, gas sensing and optical properties. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Pahlavan, A.; Karimi-Maleh, H.; Karimi, F.; Amiri, M.A.; Khoshnama, Z.; Shahmiri, M.R.; Keyvanfard, M. Application of CdO nanoparticle ionic liquid modified carbon paste electrode as a high sensitive biosensor for square wave voltammetric determination of nadh. Mater. Sci. Eng. C 2014, 45, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, Y.C.; Uhm, H.S. Direct synthesis and characterization of CdO nano-cubes. Jpn. J. Appl. Phys. 2007, 46. [Google Scholar] [CrossRef]
- Yahia, I.S.; Salem, G.F.; Abd El-sadek, M.S.; Yakuphanoglu, F. Optical properties of Al-CdO nano-clusters thin films. Superlattice Microstruct. 2013, 64, 178–184. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liang, C.H.; Wang, G.Z.; Gao, T.; Wang, S.X.; Fan, J.C.; Zhang, L.D. Preparation and characterization of ordered semiconductor cdo nanowire arrays. J. Mater. Sci. Lett. 2001, 20, 1687–1689. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Wang, X. Ordered semiconductor cdo nanowire arrays: Synthesising by one-step low-temperature electrodeposition and optical properties. Int. J. Nanotechnol. 2007, 4, 110–118. [Google Scholar] [CrossRef]
- Srinivasaraghavan, R.; Chandiramouli, R.; Jeyaprakash, B.G.; Seshadri, S. Quantum chemical studies on CdO nanoclusters stability. Spectrochim. Acta A 2013, 102, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Reza Khayati, G.; Dalvand, H.; Darezereshki, E.; Irannejad, A. A facile method to synthesis of CdO nanoparticles from spent Ni-Cd batteries. Mater. Lett. 2014, 115, 272–274. [Google Scholar] [CrossRef]
- Askarinejad, A.; Morsali, A. Synthesis of cadmium(ii) hydroxide, cadmium(ii) carbonate and cadmium(ii) oxide nanoparticles; investigation of intermediate products. Chem. Eng. J. 2009, 150, 569–571. [Google Scholar] [CrossRef]
- Choi, D.; Jeong, G.H.; Kim, S.-W. Fabrication of size and shape controlled cadmium oxide nanocrystals. Bull. Korean Chem. Soc. 2011, 32, 3851–3852. [Google Scholar] [CrossRef]
- Radi, P.; Brito-Madurro, A.; Madurro, J.; Dantas, N. Characterization and properties of CdO nanocrystals incorporated in polyacrylamide. Braz. J. Phys. 2006, 36, 412–414. [Google Scholar] [CrossRef]
- Bunge, S.D.; Krueger, K.M.; Boyle, T.J.; Rodriguez, M.A.; Headley, T.J.; Colvin, V.L. Growth and morphology of cadmium chalcogenides: The synthesis of nanorods, tetrapods, and spheres from CdO and Cd (O2CCH3)2. J. Mater. Chem. 2003, 13, 1705–1709. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Imani, M.; Kerdari, H.; Bijanzad, K.; Khaledi, D.; Rad, M. Preparation of cdo rhombus-like nanostructure and its photocatalytic degradation of azo dyes from aqueous solution. Nanomater. Nanotechnol. 2014. [Google Scholar] [CrossRef]
- Zheng, Z.; Gan, L.; Li, H.; Ma, Y.; Bando, Y.; Golberg, D.; Zhai, T. A fully transparent and flexible ultraviolet-visible photodetector based on controlled electrospun zno-cdo heterojunction nanofiber arrays. Adv. Funct. Mater. 2015, 25, 5885–5894. [Google Scholar] [CrossRef]
- Zaien, M.; Hmood, A.; Ahmed, N.M.; Hassan, Z. Growth and characterization of different structured CdO using a vapor transport. Mater. Lett. 2013, 102–103, 12–14. [Google Scholar] [CrossRef]
- Askarinejad, A.; Morsali, A. Syntheses and characterization of CdCO3 and CdO nanoparticles by using a sonochemical method. Mater. Lett. 2008, 62, 478–482. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, G.L. Solvothermal synthesis of CdO hollow nanostructures from CdO2 nanoparticles. Mater. Lett. 2008, 62, 673–675. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Sonochemical syntheses of a nanoparticles cadmium (ii) supramolecule as a precursor for the synthesis of cadmium (ii) oxide nanoparticles. Ultrason. Sonochem. 2012, 19, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Yufanyi, D.M.; Tendo, J.F.; Ondoh, A.M.; Mbadcam, J.K. CdO nanoparticles by thermal decomposition of a cadmium-hexamethylenetetramine complex. J. Mater. Sci. Res. 2014, 3. [Google Scholar] [CrossRef]
- Athar, T.; Shafi, S.; Khan, A.A. Soft chemical process for synthesis of cdo nanoparticles. Mater. Focus 2014, 3, 397–400. [Google Scholar] [CrossRef]
- Prakash, T.; Neri, G.; Kumar, E.R. A comparative study of the synthesis of CdO nanoplatelets by an albumen-assisted isothermal evaporation method. J. Alloy Compd. 2015, 624, 258–265. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, S.A. A facile thermal-treatment route to synthesize ZnO nanosheets and effect of calcination temperature. PLoS ONE 2014, 9, e103134. [Google Scholar]
- Cullity, B. Elements of X-ray Diffraction, 2nd ed.; Adisson-Wesley Publishing: Boston, MA, USA, 1978. [Google Scholar]
- Ghoshal, T.; Biswas, S.; Nambissan, P.; Majumdar, G.; de, S.K. Cadmium oxide octahedrons and nanowires on the micro-octahedrons: A simple solvothermal synthesis. Cryst. Growth Des. 2009, 9, 1287–1292. [Google Scholar] [CrossRef]
- Balu, A.; Nagarethinam, V.; Suganya, M.; Arunkumar, N.; Selvan, G. Effect of solution concentration on the structural, optical and electrical properties of silar deposited cdo thin films. J. Electron. Dev. 2012, 12, 739–749. [Google Scholar]
- Jia, Y.; Yu, X.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Shape-controlled synthesis of CdCo3 microcrystals and corresponding nanoporous cdo architectures. RSC Adv. 2012, 2, 10251–10254. [Google Scholar] [CrossRef]
- Ghosh, M.; Rao, C.N.R. Solvothermal synthesis of CdO and CuO nanocrystals. Chem. Phys. Lett. 2004, 393, 493–497. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, C. Optical properties of surface-modified cdo nanoparticles. Opt. Mater. 2003, 22, 227–233. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. A contribution to the optics of pigments. Z. Tech. Phys. 1931, 12, 593–599. [Google Scholar]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part I. JOSA 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Johnson, E.J. Absorption near the fundamental edge. Semicond. Semimet. 1967, 3, 153–258. [Google Scholar]
- Torrent, J.; Barron, V. Encyclopedia of Surface and Colloid Science; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 1438–1446. [Google Scholar]
- Pankove, J.I. Optical Processes in Semiconductors; Courier Corporation: North Chelmsford, MA, USA, 2012. [Google Scholar]
- Aita, C.R.; Liu, Y.-L.; Kao, M.L.; Hansen, S.D. Optical behavior of sputter-deposited vanadium pentoxide. J. Appl. Phys. 1986, 60, 749–753. [Google Scholar] [CrossRef]
- Xiaochun, W.; Rongyao, W.; Bingsuo, Z.; Li, W.; Shaomei, L.; Jiren, X.; Wei, H. Optical properties of nanometer-sized cdo organosol. J. Mater. Res. 1998, 13, 604–609. [Google Scholar] [CrossRef]
- Eychmüller, A. Structure and photophysics of semiconductor nanocrystals. J. Phys. Chem. B 2000, 104, 6514–6528. [Google Scholar] [CrossRef]
- Gulino, A.; Tabbì, G. CdO thin films: A study of their electronic structure by electron spin resonance spectroscopy. Appl. Surf. Sci. 2005, 245, 322–327. [Google Scholar] [CrossRef]
- Gulino, A.; Fragalà, I. Synthesis and spectroscopic characterisation of y-doped Cd2SNO4. J. Mater. Chem. 1999, 9, 2837–2841. [Google Scholar] [CrossRef]








| CdO Semiconductor NPs | PVP Concentration (g/mL) | Intensity (%) | DXRD (nm) | DTEM (nm) | Eg (eV) |
|---|---|---|---|---|---|
| CdO SNPs 1 | 0.00 | 9,636 | – | – | – |
| CdO SNPs 2 | 0.03 | 4,958 | 38 | 37 ± 3 | 2.11 |
| CdO SNPs 3 | 0.04 | 4,804 | 31 | 32 ± 2 | 2.13 |
| CdO SNPs 4 | 0.05 | 4,277 | 22 | 23 ± 3 | 2.15 |
| PVP (g/mL) | 𝑔-Factor | Hr (Oe) |
|---|---|---|
| 0.00 | – | – |
| 0.03 | 1.99513 | 328.764 |
| 0.04 | 1.99287 | 329.099 |
| 0.05 | 1.99235 | 329.294 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hada, N.M.; Saion, E.; Talib, Z.A.; Shaari, A.H. The Impact of Polyvinylpyrrolidone on Properties of Cadmium Oxide Semiconductor Nanoparticles Manufactured by Heat Treatment Technique. Polymers 2016, 8, 113. https://doi.org/10.3390/polym8040113
Al-Hada NM, Saion E, Talib ZA, Shaari AH. The Impact of Polyvinylpyrrolidone on Properties of Cadmium Oxide Semiconductor Nanoparticles Manufactured by Heat Treatment Technique. Polymers. 2016; 8(4):113. https://doi.org/10.3390/polym8040113
Chicago/Turabian StyleAl-Hada, Naif Mohammed, Elias Saion, Zainal Abidin Talib, and Abdul Halim Shaari. 2016. "The Impact of Polyvinylpyrrolidone on Properties of Cadmium Oxide Semiconductor Nanoparticles Manufactured by Heat Treatment Technique" Polymers 8, no. 4: 113. https://doi.org/10.3390/polym8040113
APA StyleAl-Hada, N. M., Saion, E., Talib, Z. A., & Shaari, A. H. (2016). The Impact of Polyvinylpyrrolidone on Properties of Cadmium Oxide Semiconductor Nanoparticles Manufactured by Heat Treatment Technique. Polymers, 8(4), 113. https://doi.org/10.3390/polym8040113