Modification of Spherical Polyelectrolyte Brushes by Layer-by-Layer Self-Assembly as Observed by Small Angle X-ray Scattering
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
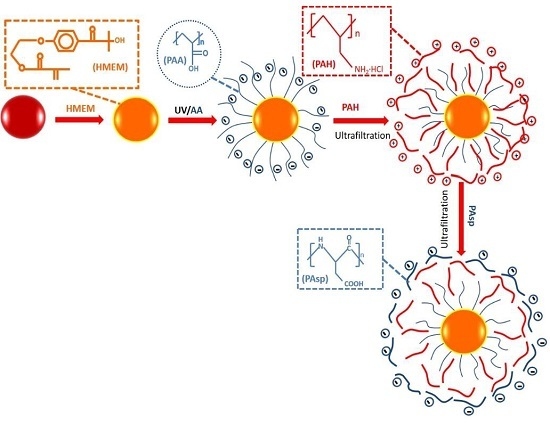
2.2. Preparation of Multilayer Modified SPBs
2.3. Characterization
2.4. SAXS Fitting Model
3. Results and Discussion
3.1. Structure of Multilayer SPB
3.1.1. By TEM
3.1.2. By Zeta Potential and DLS
3.1.3. By SAXS
3.2. Effect of pH
3.2.1. PAA-PAH Double-Layer Modified SPB
3.2.2. PAA-PAH-PAsp Triple-Layer Modified SPB
3.3. Effect of Ionic Strength
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Cock, L.J.; de Koker, S.; de Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric multilayer capsules in drug delivery. Angew. Chem. Int. Ed. 2010, 49, 6954–6973. [Google Scholar]
- Gentile, P.; Carmagnola, I.; Nardo, T.; Chiono, V. Layer-by-layer assembly for biomedical applications in the last decade. Nanotechnology 2015, 26, 422001. [Google Scholar] [CrossRef] [PubMed]
- Antipina, M.N.; Kiryukhin, M.V.; Skirtach, A.G.; Sukhorukov, G.B. Micropackaging via layer-by-layer assembly: Microcapsules and microchamber arrays. Int. Mater. Rev. 2014, 59, 224–244. [Google Scholar] [CrossRef]
- Min, K.H.; Lee, H.J.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Lee, S.C. The tumor accumulation and therapeutic efficacy of doxorubicin carried in calcium phosphate-reinforced polymer nanoparticles. Biomaterials 2012, 33, 5788–5797. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Haidar, Z.S.; Tran, T.H.; Choi, J.Y.; Jeong, J.H.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014, 10, 5116–5127. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, Z.; Feng, M. Nanocarriers with tunable surface properties to unblock bottlenecks in systemic drug and gene delivery. J. Control. Release 2015, 214, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Anandhakumar, S.; Gokul, P.; Raichur, A.M. Stimuli-responsive weak polyelectrolyte multilayer films: A thin film platform for self-triggered multi-drug delivery. Mat. Sci. Eng. C 2016, 58, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ono, T.; Kashiwagi, Y.; Kashiwagi, Y.; Takahashi, S.; Katsuhiko, S.; Anzai, J. pH-Dependent release of insulin from layer-by-layer-deposited polyelectrolyte microcapsules. Polymers 2015, 7, 1269–1278. [Google Scholar] [CrossRef]
- Oćwieja, M.; Adamczyk, Z.; Morga, M.; Kubiak, K. Influence of supporting polyelectrolyte layers on the coverage and stability of silver nanoparticle coatings. J. Colloid. Interface Sci. 2015, 445, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wytrwal, M.; Koczurkiewicz, P.; Zrubek, K.; Niemiec, W.; Michalik, M.; Kozik, B.; Szneler, E.; Bernasik, A.; Madeja, Z.; Nowakowska, M.; et al. Growth and motility of human skin fibroblasts on multilayer strong polyelectrolyte films. J. Colloid. Interface Sci. 2016, 461, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.B.; Wang, D.G.; Liu, S.H.; Zhang, S.W. Fabrication and tribological properties of polyelectrolyte multilayers containing in situ gold and silver nanoparticles. Colloids Surf. A 2013, 417, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Zacharia, N.S. Functional polyelectrolyte multilayer assemblies for surfaces with controlled wetting behavior. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, Y.; Zhang, W.; Qi, H.; Zhang, C. An amperometric glucose biosensor based on layer-by-layer GOx-SWCNT conjugate/redox polymer multilayer on a screen-printed carbon electrode. Sens. Actuators B Chem. 2011, 153, 219–225. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Evans, E.; Benavidez, T.E.; Garcia, C.D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, Z.; Zhang, X.; Chu, S.; Xu, W.; Liu, B.; Qu, C.; Xie, L.; Fan, Q.; Lai, W. Efficient blue organic light-emitting devices based on solution-processed starburst macromolecular electron injection layer. J. Lumin. 2016, 170, 50–55. [Google Scholar] [CrossRef]
- Zhao, F.; Tian, Y.; Liu, J.; Wang, J.; Zhou, Z. Preparation of Au/Ag multilayers via layer-by-layer self-assembly in spherical polyelectrolyte brushes and their catalytic activity. Chin. J. Polym. Sci. 2015, 33, 1421–1430. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.A.; Möhwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Khopade, A.J.; Caruso, F. Two-component, ultrathin microcapsules prepared by a core-mediated layer-by-layer approach. Chem. Mater. 2004, 16, 2107–2112. [Google Scholar] [CrossRef]
- Chen, M.X.; Li, B.K.; Yin, D.K.; Liang, J.; Li, S.S.; Peng, D.Y. Layer-by-layer assembly of chitosan stabilized multilayered liposomes for paclitaxel delivery. Carbohydr. Polym. 2014, 111, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Choi, M.J.; Min, S.G.; Weiss, J. Formation and stability of multiple-layered liposomes by layer-by-layer electrostatic deposition of biopolymers. Food Hydrocoll. 2013, 30, 249–257. [Google Scholar] [CrossRef]
- Becker, A.L.; Zelikin, A.N.; Johnston, A.P.R.; Caruso, F. Tuning the formation and degradation of layer-by-layer assembled polymer hydrogel microcapsules. Langmuir 2009, 25, 14079–14085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Caruso, F. Nanoporous protein particles through templating mesoporous silica spheres. Adv. Mater. 2006, 18, 795–800. [Google Scholar] [CrossRef]
- Mauser, T.; Déjugnat, C.; Möhwald, H.; Sukhorukov, G.B. Microcapsules made of weak polyelectrolytes: Templating and stimuli-responsive properties. Langmuir 2006, 22, 5888–5893. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Weiss, A.; Ballauff, M. Synthesis of spherical polyelectrolyte brushes by photoemulsion polymerization. Macromolecules 1999, 32, 6043–6046. [Google Scholar] [CrossRef]
- de Robillard, Q.; Guo, X.; Ballauff, M.; Narayanan, T. Spatial correlation of spherical polyelectrolyte brushes in salt-free solution as observed by small-angle X-ray scattering. Macromolecules 2000, 33, 9109–9114. [Google Scholar] [CrossRef]
- Stieler, T.; Scholle, F.D.; Weiss, A.; Ballauff, M.; Kaatze, U. Ultrasonic spectrometry of polystyrene latex suspensions. Scattering and configurational elasticity of polymer chains. Langmuir 2001, 17, 1743–1751. [Google Scholar] [CrossRef]
- Guo, X.; Ballauff, M. Spherical polyelectrolyte brushes: Comparison between annealed and quenched brushes. Phys. Rev. E 2001, 64, 051406. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Guo, X.; Ballauff, M. The osmotic coefficient of spherical polyelectrolyte brushes in aqueous salt-free solution. Mol. Organ. Interfaces 2002, 121, 34–38. [Google Scholar]
- Ballauff, M. Nanoscopic polymer particles with a well-defined surface: Synthesis, characterization, and properties. Macromol. Chem. Phys. 2003, 204, 220–234. [Google Scholar] [CrossRef]
- Sharma, G.; Ballauff, M. Cationic spherical polyelectrolyte brushes as nanoreactors for the generation of gold particles. Macromol. Rapid Commun. 2004, 25, 547–552. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Drechsler, M.; Ballauff, M. Thermosensitive core-shell particles as carriers for Ag nanoparticles: Modulating the catalytic activity by a phase transition in networks. Angew. Chem. Int. Ed. 2006, 45, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Schrinner, M.; Proch, S.; Mei, Y.; Kempe, R.; Miyajima, N.; Ballauff, M. Stable bimetallic gold-platinum nanoparticles immobilized on spherical polyelectrolyte brushes: Synthesis, characterization, and application for the oxidation of alcohols. Adv. Mater. 2008, 20, 1928–1933. [Google Scholar] [CrossRef]
- Wittemann, A.; Haupt, B.; Ballauff, M. Adsorption of proteins on spherical polyelectrolyte brushes in aqueous solution. Phys. Chem. Chem. Phys. 2003, 5, 1671–1677. [Google Scholar] [CrossRef]
- Henzler, K.; Wittemann, A.; Breininger, E.; Ballauff, M.; Rosenfeldt, S. Adsorption of bovine hemoglobin onto spherical polyelectrolyte brushes monitored by small-angle X-ray scattering and Fourier transform infrared spectroscopy. Biomacromolecules 2007, 8, 3674–3681. [Google Scholar] [CrossRef] [PubMed]
- Henzler, K.; Haupt, B.; Lauterbach, K.; Wittemann, A.; Borisov, O.; Ballauff, M. Adsorption of beta-lactoglobulin on spherical polyelectrolyte brushes: Direct proof of counterion release by isothermal titration calorimetry. J. Am. Chem. Soc. 2010, 132, 3159–3163. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, K.; Xu, Y.; Yu, X.; AWang, W.; Li, L.; Guo, X. Protein immobilization and separation using anionic/cationic spherical polyelectrolyte brushes based on charge anisotropy. Soft Matter 2013, 9, 11276–11287. [Google Scholar] [CrossRef]
- Lu, Y.; Hoffmann, M.; Yelamanchili, R.S.; Terrenoire, A.; Schirinner, M.; Drechsler, M.; Möller, M.W.; Breu, J.; Ballauff, M. Well-defined crystalline TiO2 nanoparticles generated and immobilized on a colloidal nanoreactor. Macromol. Chem. Phys. 2009, 210, 377–386. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Bouropoulos, N.; Zboril, R.; Lliopoulos, K.; Boukos, N.; Chatzikyriakos, G.; Couris, S. Optically active spherical polyelectrolyte brushes with a nanocrystalline magnetic core. Adv. Funct. Mater. 2008, 18, 1694–1706. [Google Scholar] [CrossRef]
- Huang, S.; Yu, X.; Dong, Y.; Li, L.; Guo, X. Spherical polyelectrolyte brushes: Ideal templates for preparing pH-sensitive core-shell and hollow silica nanoparticles. Colloids Surf. A 2012, 415, 22–30. [Google Scholar] [CrossRef]
- Varga, N.; Benkő, M.; Sebők, D.; Dékány, I. BSA/polyelectrolyte core-shell nanoparticles for controlled of encapsulated ibuprofen. Colloids Surf. B 2014, 123, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Tangso, K.J.; Patel, H.; Lindberg, S.; Hartley, P.G.; Knott, R.; Spicer, P.T.; Boyd, B.J. Controlling the mesostructure formation within the shell of novel cubic/hexagonal phase cetyltrimethylammonium bromide-poly(acrylamide-acrylic acid) capsules for pH stimulated release. ACS Appl. Mater. Interface 2015, 7, 24501–24509. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, T.A.; Lassenberger, A.; van Oostrum, P.D.J.; Rennhofer, H.; Zirbs, R.; Capone, B.; Vonderhaid, I.; Amenitsch, H.; Lichtenegger, H.C.; Reimhult, E. Core-shell structure of monodisperse poly(ethylene glycol)-grafted iron oxide nanoparticles studied by small-angle x-ray scattering. Chem. Mater. 2015, 27, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, L.; Yu, X.; Han, H.; Guo, X. Distribution of magnetic nanoparticles in spherical polyelectrolyte brushes as observed by small-angle X-ray scattering. J. Polym. Sci. A Polym. Phys. 2014, 52, 1681–1688. [Google Scholar]
- Wang, W.; Li, L.; Han, H.; Tian, Y.; Zhou, Z.; Guo, X. Tunable immobilization of protein in anionic spherical polyelectrolyte brushes as observed by small-angle X-ray scattering. Colloid Polym. Sci. 2015, 293, 2789–2798. [Google Scholar] [CrossRef]
- Xia, B.; Mamonov, A.; Leysen, S.; Allen, K.N.; Strelkov, S.V.; Paschalidis, L.C.; Vajda, S.; Kozakov, D. Accounting for observed small angle X-ray scattering profile in the protein–protein docking server cluspro. J. Comput. Chem. 2015, 36, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Müller-Buschbaum, P.; Gutmann, J.S.; Stamm, M. Dewetting of confined polymer films: An X-ray and neutron scattering study. Phys. Chem. Chem. Phys. 1999, 1, 3857–3863. [Google Scholar] [CrossRef]
- Wang, W.; Chu, F.; Li, L.; Han, H.; Tian, Y.; Wang, Y.; Yuan, Z.; Zhou, Z.; Guo, X. Interactions among spherical poly(acrylic acid) brushes: Observation by rheology and small angle X-ray scattering. J Polym. Sci. B Polym. Phys. 2016, 54, 405–413. [Google Scholar] [CrossRef]
- Dingenouts, N.; Norhausen, C.; Ballauff, M. Observation of the volume transition in thermosensitive core-shell latex particles by small-angle X-ray scattering. Macromolecules 1998, 31, 8912–8917. [Google Scholar] [CrossRef]
- Hatto, N.; Cosgrove, T.; Snowden, M.J. Novel microgel-particle colloids: The detailed characterisation of the layer structure and chain topology of silica: Poly(NIPAM) core-shell particles. Polymer 2000, 41, 7133–7137. [Google Scholar] [CrossRef]
- Seelenmeyer, S.; Deike, I.; Rosenfeldt, S.; Norhausen, C.; Dingenouts, N.; Ballauff, M.; Narayanan, T.; Lindner, P. Small-angle X-ray and neutron scattering studies of the volume phase transition in thermosensitive core–shell colloids. J. Chem. Phys. 2001, 114, 10471–10478. [Google Scholar] [CrossRef]
- Rosenfeldt, S.; Wittemann, A.; Ballauff, M.; Breininger, E.; Bolze, J.; Dingenouts, N. Interaction of proteins with spherical polyelectrolyte brushes in solution as studied by small-angle X-ray scattering. Phys. Rev. E 2004, 70, 061403. [Google Scholar] [CrossRef] [PubMed]
- Henzler, K.; Rosenfeldt, S.; Wittemann, A.; Harnau, L.; Finet, S.; Narayanan, T.; Ballauff, M. Directed motion of proteins along tethered polyelectrolytes. Phys. Rev. Lett. 2008, 100, 158301. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jain, S.; Prasad, K.N.; Jain, S.K.; Vyas, S.P. Polyelectrolyte coated multilayered liposomes (nanocapsules) for the treatment of Helicobacter pylori infection. Mol. Pharm. 2009, 6, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Gao, C. Stable microcapsules assembled stepwise from weak polyelectrolytes followed by thermal crosslinking. Polym. Adv. Technol. 2005, 16, 827–833. [Google Scholar] [CrossRef]











| Substance | ρ (g/cm3) | ρi (e/nm3) β | Δρ (ρi-ρH2O, e/nm3) |
|---|---|---|---|
| H2O | 0.997 * | 333.3 | 0 |
| Poly(styrene) | 1.05 * | 339.7 | 6.4 |
| Poly(arylic acid) | 1.19 α | 377.9 | 44.6 |
| Poly(allylamine hydrochloride) | 1.10 α | 376.8 | 43.5 |
| poly-l-aspartic acid | 1.20 α | 376.9 | 43.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, L.; Han, H.; Wang, W.; Wang, Y.; Ye, Z.; Guo, X. Modification of Spherical Polyelectrolyte Brushes by Layer-by-Layer Self-Assembly as Observed by Small Angle X-ray Scattering. Polymers 2016, 8, 145. https://doi.org/10.3390/polym8040145
Tian Y, Li L, Han H, Wang W, Wang Y, Ye Z, Guo X. Modification of Spherical Polyelectrolyte Brushes by Layer-by-Layer Self-Assembly as Observed by Small Angle X-ray Scattering. Polymers. 2016; 8(4):145. https://doi.org/10.3390/polym8040145
Chicago/Turabian StyleTian, Yuchuan, Li Li, Haoya Han, Weihua Wang, Yunwei Wang, Zhishuang Ye, and Xuhong Guo. 2016. "Modification of Spherical Polyelectrolyte Brushes by Layer-by-Layer Self-Assembly as Observed by Small Angle X-ray Scattering" Polymers 8, no. 4: 145. https://doi.org/10.3390/polym8040145
APA StyleTian, Y., Li, L., Han, H., Wang, W., Wang, Y., Ye, Z., & Guo, X. (2016). Modification of Spherical Polyelectrolyte Brushes by Layer-by-Layer Self-Assembly as Observed by Small Angle X-ray Scattering. Polymers, 8(4), 145. https://doi.org/10.3390/polym8040145