Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Tensile Properties Testing
2.4. Film Characterization
2.4.1. Morphological Studies
2.4.2. Determination of Drug Loading
2.4.3. Water Content Determination
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.7. In Vitro Indomethacin Release
- (a)
- Zero-order kinetics: The release rate data were fitted into the following equation,where Qt is the amount of drug dissolved in time (t), Qo is the initial amount of drug in the solution, and Ko is the zero-order release constant.
- (b)
- Higuchi matrix model: The release rate data were fitted to the following equation,where Qt is the amount of drug released in time (t), KH is the Higuchi diffusion constant.
- (c)
- Korsmeyer–Peppas empirical power law:where, Mt/M8 is the fraction of drug released at a time (t), K is the structural and geometrical constant and n is the release exponent.
2.8. Statistical Analysis
3. Results and Discussion
3.1. Film Preparation
3.2. LMP Films Characterization
3.3. Mechanical Properties
3.3.1. Tensile Strength at Break of Pectin Plasticized Films
3.3.2. Elongation at Break of Pectin Plasticized Films
3.3.3. Young’s Modulus of Pectin Plasticized Films
3.3.4. Mechanical Properties of Pectin-Loaded Films
3.4. DSC Analysis
3.5. FT-IR Spectroscopic Study
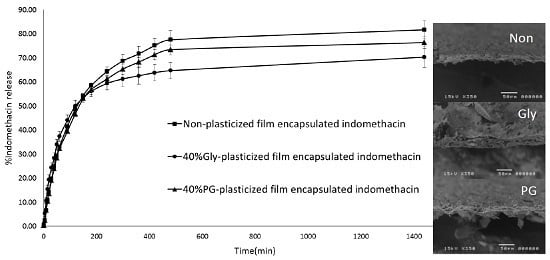
3.6. In Vitro Release of Indomethacin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alves, V.D.; Costa, N.; Coelhoso, I.M. Barrier properties of biodegradable composite films based on kappa-carrageenan/pectin blends and mica flakes. Carbohydr. Polym. 2010, 79, 269–276. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Krochta, M.; Johnston, C.D. Edible and biodegradable polymer films: Challenges and opportunities. Food Technol. 1997, 51, 61–74. [Google Scholar]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D.A. Pectins: Structure, biosyntbesis and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–961. [Google Scholar] [CrossRef]
- Rolin, C. Pectin. In Industril Gums: Polysaccharides and Their Derivatives; Whistler, R.L., Bemiller, J.N., Eds.; Academic Press: New York, NY, USA, 1993; pp. 257–293. [Google Scholar]
- Seixasa, F.L.; Turbianib, F.R.B.; Salomãob, P.G.; Souzaa, R.P.; Gimenes, M.L. Biofilms composed of alginate and pectin: Effect of concentration of crosslinker and plasticizer agents. Chem. Eng. Trans. 2013, 32, 1693–1698. [Google Scholar]
- Cabello, P.S.D.; Takara, E.A.; Marchese, J.; Ochoa, N.A. Influence of plasticizers in pectin films: Microstructural changes. Mater. Chem. Phys. 2015, 162, 491–497. [Google Scholar] [CrossRef]
- Kwan, K.C.; Breault, G.O.; Umbenhauer, E.R.; McMahon, F.G.; Duggan, D.E. Kinetics of indomethacin absorption, elimination, and enterohepatic circulation in man. Eur. J. Pharm. Biopharm. 1976, 4, 255–280. [Google Scholar] [CrossRef]
- Deore, V.A.; Kumar, R.S.; Gide, P.S. Development and statistical optimization of mucoadhesive buccal patches of indomethacin: In Vitro and ex vivo evaluation. Int. J. Adv. Pharm. Biol. Chem. 2013, 2, 405–422. [Google Scholar]
- Puglia, C.; Trombetta, D.; Venuti, V.; Saija, A.; Bonina, F. Evaluation of in vitro topical anti-inflammatory activity of indomethacin from liposomal vesicles. J. Pharm. Pharmacol. 2004, 56, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Takahashi, A.; Kubo, W.; Bachynsky, J.; Lobenberg, R. Poly N-butylcyanoacrylate (PNBCA) nanocapsules as a carrier for NSAIDs: In vitro release and in vivo skin penetration. J. Pharm. Pharm. Sci. 2003, 6, 238–245. [Google Scholar] [PubMed]
- Pérez, C.D.; Flores, S.K.; Marangoni, A.G.; Gerschenson, L.N.; Rojas, A.M. Development of a high methoxyl pectin edible film for retention of l-(+)-ascorbic acid. J. Agric. Food. Chem. 2009, 57, 6844–6855. [Google Scholar] [CrossRef] [PubMed]
- Hermans, K.; Van Den Plas, D.; Kerimova, S.; Carleer, R.; Adriaensens, P.; Weyenberg, W.; Ludwig, A. Development and characterization of mucoadhesive chitosan films for ophthalmic delivery of cyclosporine A. Int. J. Pharm. 2014, 472, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Krochta, J.M. Proteins as raw materials for films and coatings: Definitions, current status, and opportunities. In Protein-Based Films and Coatings; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–42. [Google Scholar]
- Hiorth, M.; Tho, I.; Sande, S.A. The formation and permeability of drugs across free pectin and chitosan films prepared by a spraying method. Eur. J. Pharm. Biopharm. 2003, 56, 175–181. [Google Scholar] [CrossRef]
- Hoagland, P.D.; Parris, N. Chitosan/pectin laminated films. J. Agric. Food Chem. 1996, 44, 1915–1919. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; Sobral, P.J.D.A.; Menegalli, F.C. Effect of drying conditions and plasticizer type on some physical and mechanical properties of amaranth flour films. LWT Food. Sci. Technol. 2013, 50, 392–400. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Amini, A.M.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food. Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of pectin/starch blends plasticized with glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Bharkatiya, M.; Nema, R.K.; Bhatnagar, M. Designing and characterization of drug free patches for transdermal application. Int. J. Pharm. Sci. Drug Res. 2010, 2, 35–39. [Google Scholar]
- Omelczuk, M.O.; McGinity, J.W. The influence of polymer glass transition temperature and molecular weight on drug release from tablets containing poly(dl)-lactic acid. Pharm. Res. 1992, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Rowe, R.C. The Young’s modulus of pharmaceutical materials. Int. J. Pharm. 1987, 37, 15–18. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H. The influence of storage conditions heat and humidity on conformation, state transitions and degradation behavior of dried pectins. Food. Hydrocoll. 2009, 23, 856–866. [Google Scholar] [CrossRef]
- Iijima, M.; Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41, 101–105. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biological; Merck and Co., Inc.: Kenilworth, NJ, USA, 1996; p. 852. [Google Scholar]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions: The egg box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Umeda, Y.; Fukami, T.; Furuishi, T.; Suzuki, T.; Makimura, M.; Tomono, K. Molecular complex consisting of two typical external medicines: Intermolecular interaction between indomethacin and lidocaine. Chem. Pharm. Bull. 2007, 55, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, M.; Cebadera, E.; Gutiérrez, S.; Martín, C.; Montero, M.J.; Rives, V.; Rocha, J.; Evilla, M.A. Mg, Al layered double hydroxides with intercalated indomethacin: Synthesis, characterization, and pharmacological study. J. Pharm. Sci. 2004, 93, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Malik, K.; Singh, I.; Arora, S.; Rana, V. Formulation and evaluation of controlled release matrix mucoadhesive tablets of domperidone using Salvia plebeian gum. J. Adv. Pharm. Technol. Res. 2011, 2, 163–169. [Google Scholar] [PubMed]




| Indomethacin Loaded LMP Films | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) | Drug Loading (%) | Water Content (%) |
|---|---|---|---|---|---|
| Non-plasticized | 2.57 ± 0.62 | 10.91 ± 1.14 | 21.80 ± 2.38 | 103.59 ± 6.56 | 9.05 ± 0.36 |
| 40% Gly-plasticized | 3.26 ± 0.08 | 26.50 ± 3.86 | 23.41 ± 4.71 | 100.96 ± 10.10 | 9.54 ± 1.83 |
| 40% PG-plasticized | 4.41 ± 0.45 | 21.17 ± 2.27 | 32.44 ± 5.35 | 100.83 ± 9.80 | 9.60 ± 1.86 |
| Sample | Water Evaporation Peak | Indomethacin Crystalline Melting Peak | ||
|---|---|---|---|---|
| ΔH (J/g) | EPT (°C) | ΔH (J/g) | EPT (°C) | |
| Non-plasticized film | 238.8 ± 1.8 | 116.8 ± 2.7 | - | - |
| 40% Gly-plasticized film | 371.2 ± 7.6 | 120.3 ± 3.9 | - | - |
| 40% PG-plasticized film | 390.4 ± 6.2 | 111.9 ± 6.6 | - | - |
| Non-plasticized film containing indomethacin | 191.6 ± 12.3 | 109.5 ± 1.1 | 39.9 ± 1.6 | 159.9 ± 0.6 |
| 40% Gly-plasticized film containing indomethacin | 235.3 ± 18.3 | 114.4 ± 0.3 | 21.3 ± 4.5 | 158.0 ± 1.2 |
| 40% PG-plasticized film containing indomethacin | 310.6 ± 19.9 | 119.2 ± 0.1 | 25.7 ± 2.2 | 160.0 ± 0.3 |
| Indomethacin | - | - | 106.4 ± 0.4 | 161.9 ± 0.3 |
| Sample | Zero-Order | Higuchi | Korsmeyer–Peppas | |||
|---|---|---|---|---|---|---|
| r2 | K0 (h−1) | r2 | KH (h1/2) | r2 | n | |
| Non-plasticized film containing indomethacin | 0.9491 | 4.4360 | 0.9985 | 9.8634 | 0.9659 | 0.6921 |
| 40% Gly-plasticized film containing indomethacin | 0.9098 | 8.3395 | 0.9910 | 4.781 | 0.9732 | 0.8175 |
| 40% PG-plasticized film containing indomethacin | 0.9477 | 4.4795 | 0.9977 | 9.2185 | 0.9694 | 0.8881 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers 2017, 9, 289. https://doi.org/10.3390/polym9070289
Jantrawut P, Chaiwarit T, Jantanasakulwong K, Brachais CH, Chambin O. Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers. 2017; 9(7):289. https://doi.org/10.3390/polym9070289
Chicago/Turabian StyleJantrawut, Pensak, Tanpong Chaiwarit, Kittisak Jantanasakulwong, Claire Hélène Brachais, and Odile Chambin. 2017. "Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin" Polymers 9, no. 7: 289. https://doi.org/10.3390/polym9070289
APA StyleJantrawut, P., Chaiwarit, T., Jantanasakulwong, K., Brachais, C. H., & Chambin, O. (2017). Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers, 9(7), 289. https://doi.org/10.3390/polym9070289