Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Arsenic and Main Nutrient Contents
2.3. Preparation of Crude Extracts for Enzyme and Lipid Peroxidation Assays
2.4. Enzyme Activity Assays
2.5. Detection and Quantification of Ascorbate, GSH and GSSG by Liquid Chromatography-Electrospray Mass Spectrometry (LC-ES/MS)
2.6. Other Assays
2.7. Statistical Analysis
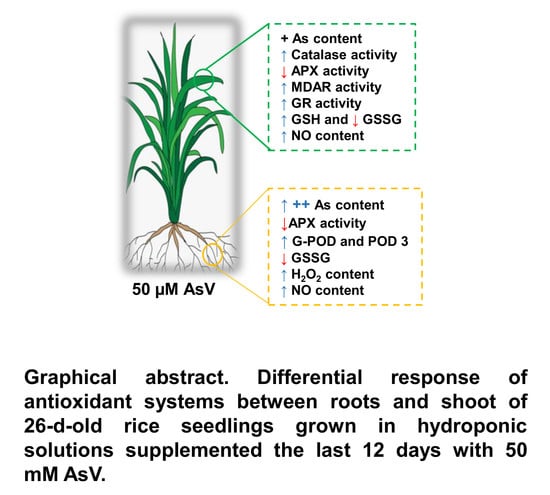
3. Results
4. Discussion
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Torres, C.; Feriche-Linares, R.; Rodríguez-Ruíz, M.; Palma, J.M.; Corpas, F.J. Arsenic-induced stress activates sulfur metabolism in different organs of garlic (Allium sativum L.) plants accompanied by a general decline of the NADPH-generating systems in roots. J. Plant Physiol. 2017, 211, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Tahir, N. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ruiz, M.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Alam, M.Z.; Hoque, M.A.; Ahammed, G.J.; McGee, R.; Carpenter-Boggs, L. Arsenic accumulation in lentil (Lens culinaris) genotypes and risk associated with the consumption of grains. Sci. Rep. 2019, 9, 9431. [Google Scholar] [CrossRef] [Green Version]
- Ali, W.; Zhang, H.; Junaid, M.; Mao, K.; Xu, N.; Chang, C.Y.; Rasool, A.; Aslam, M.; Ali, J.; Yang, Z.G. Insights into the mechanisms of arsenic-selenium interactions and the associated toxicity in plants, animals, and humans: A critical review. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Ventura-Lima, J.; Bogo, M.R.; Monserrat, J.M. Arsenic toxicity in mammals and aquatic animals: A comparative biochemical approach. Ecotoxicol. Environ. Saf. 2011, 74, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Abdul, K.S.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Escudero-Lourdes, C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology 2016, 53, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110. [Google Scholar] [CrossRef]
- Islam, E.; Khan, M.T.; Irem, S. Biochemical mechanisms of signaling: Perspectives in plants under arsenic stress. Ecotoxicol. Environ. Saf. 2015, 114, 126–133. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Arsenic uptake and accumulation mechanisms in rice species. Plant 2020, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Song, W.Y.; Mendoza-Cózatl, D.G.; Lee, Y.; Schroeder, J.I.; Ahn, S.N.; Lee, H.S.; Wicker, T.; Martinoia, E. Phytochelatin-metal(loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Environ. 2014, 37, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Chen, Y.; Miller, A.J.; Zhao, F.J. The C-type ATP-binding cassette transporter OsABCC7 is involved in the root-to-shoot translocation of arsenic in rice. Plant Cell Physiol. 2019, 60, 1525–1535. [Google Scholar] [CrossRef]
- Schmöger, M.E.; Oven, M.; Grill, E. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 2000, 122, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.A.; Chen, A.; Schroeder, J.I. ars1, an Arabidopsis mutant exhibiting increased tolerance to arsenate and increased phosphate uptake. Plant J. 2003, 35, 637–646. [Google Scholar] [CrossRef]
- Quaghebeur, M.; Rengel, Z. Arsenic uptake, translocation and speciation in pho1 and pho2 mutants of Arabidopsis thaliana. Physiol. Plant. 2004, 120, 280–286. [Google Scholar] [CrossRef]
- Li, Y.; Dhankher, O.P.; Carreira, L.; Lee, D.; Chen, A.; Schroeder, J.I.; Balish, R.S.; Meagher, R.B. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004, 45, 1787–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, G.P.; Jahn, T.P. Major intrinsic proteins and arsenic transport in plants: New players and their potential role. Adv. Exp. Med. Biol. 2010, 679, 111–125. [Google Scholar]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Barroso, J.B.; Palma, J.M.; Corpas, F.J. Cytosolic NADP-isocitrate dehydrogenase in Arabidopsis leaves and roots. Biol. Plant. 2012, 56, 705–710. [Google Scholar] [CrossRef]
- Corpas, F.J.; Aguayo-Trinidad, S.; Ogawa, T.; Yoshimura, K.; Shigeoka, S. Activation of NADPH-recycling systems in leaves and roots of Arabidopsis thaliana under arsenic-induced stress conditions is accelerated by knock-out of Nudix hydrolase 19 (AtNUDX19) gene. J. Plant Physiol. 2016, 192, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Han, Y.S.; Seong, H.J.; Ahn, J.S.; Nam, I.H. Arsenic uptake and speciation in Arabidopsis thaliana under hydroponic conditions. Chemosphere 2016, 154, 283–288. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, S.; Pinkas, D.; Lipavská, H. Multi-Component antioxidative system and robust carbohydrate status, the essence of plant arsenic tolerance. Antioxidants 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.; Tripathi, R.D.; Dwivedi, S.; Tripathi, P.; Dixit, G.; Sharma, Y.K.; Trivedi, P.K.; Corpas, F.J.; Barroso, J.B.; Chakrabarty, D. Arsenate and arsenite exposure modulate antioxidants and amino acids in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes. J. Hazard. Mater. 2013, 262, 1123–1131. [Google Scholar] [CrossRef]
- Farooq, M.A.; Li, L.; Ali, B.; Gill, R.A.; Wang, J.; Ali, S.; Gill, M.B.; Zhou, W. Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ. Sci. Pollut. Res. Int. 2015, 22, 10699–10712. [Google Scholar] [CrossRef]
- Gautam, A.; Pandey, A.K.; Dubey, R.S. Azadirachta indica and Ocimum sanctum leaf extracts alleviate arsenic toxicity by reducing arsenic uptake and improving antioxidant system in rice seedlings. Physiol. Mol. Biol. Plants 2020, 26, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Liu, W.; Chen, X.; Hu, Y.; Zhu, Y. Association of arsenic with nutrient elements in rice plants. Metallomics 2013, 5, 784–792. [Google Scholar] [CrossRef]
- Dave, R.; Singh, P.K.; Tripathi, P.; Shri, M.; Dixit, G.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Sharma, Y.K.; Dhankher, O.P.; et al. Arsenite tolerance is related to proportional thiolic metabolite synthesis in rice (Oryza sativa L.). Arch. Environ. Contam. Toxicol. 2013, 64, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Tie, B.; Zeng, M.; Qing, P.; Song, Z.; Williams, P.N.; Huang, Y. An arsenic-contaminated field trial to assess the uptake and translocation of arsenic by genotypes of rice. Environ. Geochem. Health 2013, 35, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, R.D.; Singh, R.P.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Adhikari, B. Arsenite tolerance in rice (Oryza sativa L.) involves coordinated role of metabolic pathways of thiols and amino acids. Environ. Sci. Pollut. Res. Int. 2013, 20, 884–896. [Google Scholar] [CrossRef]
- Batista, B.L.; Nigar, M.; Mestrot, A.; Rocha, B.A.; Barbosa Junior, F.; Price, A.H.; Raab, A.; Feldmann, J. Identification and quantification of phytochelatins in roots of rice to long-term exposure: Evidence of individual role on arsenic accumulation and translocation. J. Exp. Bot. 2014, 65, 1467–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, M.C.; Islam, M.S.; Islam, M.; Amin, R.; Parvez, M.S.; Kabir, A.H. Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol. Biochem. 2016, 104, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Y.H.; Cao, Y.; Zhu, Y.G.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalita, J.; Pradhan, A.K.; Shandilya, Z.M.; Tanti, B. Arsenic stress responses and tolerance in rice: Physiological, cellular and molecular approaches. Rice Sci. 2018, 25, 235–249. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.K.; Singh, D.P. Regulation of oxidative stress and mineral nutrient status by selenium in arsenic treated crop plant Oryza sativa. Ecotoxicol. Environ. Saf. 2018, 148, 105–113. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Freschi, L.; Rodríguez-Ruiz, M.; González-Gordo, S.; Corpas, F.J. Nitric oxide in the physiology and quality of fleshy fruits. J. Exp. Bot. 2019, 70, 4405–4417. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Asada, K. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: Hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol. 1996, 36, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol. 1984, 25, 1285–1295. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplast and its participation in regeneration of ascorbate for scavenging of hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Edwards, E.A.; Rawsthone, S.; Mullineaux, P.M. Subcellular distribution ofmultiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Quessada, M.P.; Macheix, J.J. Caractérisation d’une peroxydase impliquée spécifiquement dans la lignification, en relation avec l’incompatibilité au greffage chez l’Abricotier. Physiol. Végét. 1984, 22, 533–540. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Abdelly, C.; Corpas, F.J. Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 2016, 253, 885–894. [Google Scholar] [CrossRef]
- Pinilla, M.; Iglesias-Moya, J.; Campos, M.J.; Corpas, F.J.; Palma, J.M. Pomegranate (Punica granatum L.) Fruits: Characterization of the main enzymatic antioxidants (peroxisomal catalase and SOD isozymes) and the NADPH-regenerating system. Agronomy 2019, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Ádám, A.L.; Bestwick, C.S.; Barna, B.; Mansfield, J.W. Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. phaseolicola. Planta 1995, 197, 240–249. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Airaki, M.; Sánchez-Moreno, L.; Leterrier, M.; Barroso, J.B.; Palma, J.M.; Corpas, F.J. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant Cell Physiol. 2011, 52, 2006–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Ruiz, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 2017, 12, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Chaki, M.; Álvarez de Morales, P.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, J.M.; Garrido, M.; Rodríguez-García, M.I.; del Río, L.A. Peroxisome proliferation and oxidative stress mediated by activated oxygen species in plant peroxisomes. Arch. Biochem. Biophys. 1991, 287, 68–74. [Google Scholar] [CrossRef]
- Chandrakar, V.; Naithani, S.C.; Keshavkant, S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: A review. Biologia 2016, 71, 367–377. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Arvin, M.J.; Ashraf, M. Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. J. Soil Sci. Plant Nutr. 2020, 2, 577–588. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Majumder, B.; Das, S.; Mukhopadhyay, S.; Biswas, A.K. Identification of arsenic-tolerant and arsenic-sensitive rice (Oryza sativa L.) cultivars on the basis of arsenic accumulation assisted stress perception, morpho-biochemical responses, and alteration in genomic template stability. Protoplasma 2019, 256, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Otero, X.L.; Atiaga, O.; Estrella, R.; Tierra, W.; Ruales, J.; Zayas, L.; Souza, V., Jr.; Ferreira, T.O.; Nóbrega, G.N.; Oliveira, D.P.; et al. Geographical variations in arsenic contents in rice plants from Latin America and the Iberian Peninsula in relation to soil conditions. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.R.; Das, A.; Joardar, M.; De, A.; Mridha, D.; Das, R.; Rahman, M.M.; Roychowdhury, T. Flow of arsenic between rice grain and water: Its interaction, accumulation and distribution in different fractions of cooked rice. Sci. Total Environ. 2020, 731, 138937. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Kong, M.S.; Lee, B.R.; Kim, T.H.; Chae, M.J.; Lee, E.J.; Jung, G.B.; Lee, C.H.; Sung, J.K.; Kim, Y.H. Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate-glutathione homeostasis in rice seedlings. Front. Plant Sci. 2019, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Kumwimba, M.N.; Zeng, X.; Bai, L.; Wang, J. A preliminary study on genetic variation of arsenic concentration in 32 different genotypes of leafy vegetable. Environ. Pollut. 2014, 3, 72–87. [Google Scholar] [CrossRef] [Green Version]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4198–4220. [Google Scholar] [CrossRef] [Green Version]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 1–194. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; González-Gordo, S.; Cañas, A.; Campos, M.J.; Paradela, A.; Corpas, F.J.; Palma, J.M. Sweet pepper (Capsicum annuum L.) fruits contain an atypical peroxisomal catalase that is modulated by reactive oxygen and nitrogen species. Antioxidants 2019, 8, 374. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Kidwai, M.; Dhar, Y.V.; Gautam, N.; Tiwari, M.; Ahmad, I.Z.; Asif, M.H.; Chakrabarty, D. Oryza sativa class HI peroxidase (OsPRX38) overexpression in Arabidopsis thaliana reduces arsenic accumulation due to apoplastic lignification. J. Hazard. Mater. 2019, 362, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.K.; Menezes-Benavente, L.; Margis, R.; Margis-Pinheiro, M. Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: Inferences from the rice genome. J. Mol. Evol. 2004, 59, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Martins, M.O.; Ribeiro, C.W.; Fontenele, A.V.; Carvalho, F.E.; Margis-Pinheiro, M.; Silveira, J.A. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 2011, 34, 1705–1722. [Google Scholar] [CrossRef]
- Gupta, D.K.; Tripathi, R.D.; Mishra, S.; Srivastava, S.; Dwivedi, S.; Rai, U.N.; Yang, X.E.; Huanji, H.; Inouhe, M. Arsenic accumulation in root and shoot vis-a-visits effects on growth and level of phytochelatins in seedlings of Cicer arietinum L. J. Environ. Biol. 2008, 29, 281–286. [Google Scholar]
- Kim, D.Y.; Park, H.; Lee, S.H.; Koo, N.; Kim, J.G. Arsenate tolerance mechanism of Oenothera odorata from a mine population involves the induction of phytochelatins in roots. Chemosphere 2009, 75, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, W.; Guo, J.; He, Z.; Ma, M. Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings. Plant Sci. 2005, 169, 1059–1065. [Google Scholar] [CrossRef]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Yu, M.; Lamattina, L.; Spoel, S.H.; Loake, G.J. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol. 2014, 202, 1142–1156. [Google Scholar] [CrossRef]
- Corpas, F.J.; Palma, J.M. Nitric oxide on/off in fruit ripening. Plant Biol. (Stuttg.) 2018, 20, 805–807. [Google Scholar] [CrossRef]
- Takahashi, M.; Morikawa, H. Nitrate, but not nitrite, derived from nitrogen dioxide accumulates in Arabidopsis leaves following exposure to 15N-labeled nitrogen dioxide. Plant Signal. Behav. 2019, 14, 1559579. [Google Scholar] [CrossRef] [Green Version]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaki, M.; Fernández-Ocaña, A.M.; Valderrama, R.; Carreras, A.; Esteban, F.J.; Luque, F.; Gómez-Rodríguez, M.V.; Begara-Morales, J.C.; Corpas, F.J.; Barroso, J.B. Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower-mildew interaction. Plant Cell Physiol. 2009, 50, 265–279. [Google Scholar] [CrossRef] [Green Version]
- León, J.; Castillo, M.C.; Coego, A.; Lozano-Juste, J.; Mir, R. Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J. Exp. Bot. 2014, 65, 907–921. [Google Scholar] [CrossRef]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. Mechanical wounding promotes local and long distance response in the halophyte Cakile maritima through the involvement of the ROS and RNS metabolism. Nitric Oxide 2018, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Laspina, N.V.; Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005, 169, 323–330. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, Z.M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol. 2005, 46, 1915–1923. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Chakrabarty, D.; Mallick, S.; Pandey, V.; et al. Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front. Plant Sci. 2016, 6, 1272. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Alam, P.; Balawi, T.H.; Altalayan, F.H.; Ahanger, M.A.; Ashraf, A. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 2020, 244, 125480. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A.; Pandey, A.; Gupta, M. Nitric oxide alters nitrogen metabolism and PIN gene expressions by playing protective role in arsenic challenged Brassica juncea L. Ecotoxicol. Environ. Saf. 2019, 176, 95–107. [Google Scholar] [CrossRef]
- Praveen, A.; Gupta, M. Nitric oxide confronts arsenic stimulated oxidative stress and root architecture through distinct gene expression of auxin transporters, nutrient related genes and modulates biochemical responses in Oryza sativa L. Environ. Pollut. 2018, 240, 950–962. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. (Eds.) Nitric Oxide and Hydrogen Peroxide in Higher Plants; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Terrón-Camero, L.C.; Peláez-Vico, M.A.; Del-Val, C.; Sandalio, L.M.; Romero-Puertas, M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, D.; Corpas, F.J.; D’Angeli, S.; Altamura, M.M.; Falasca, G. Cadmium and arsenic-induced-stress differentially modulates Arabidopsis root architecture, peroxisome distribution, enzymatic activities and their nitric oxide content. Plant Physiol. Biochem. 2020, 148, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.Y.; Wan, Y.N.; Yu, Y.; Wang, Q.; Wang, K.; Li, H.F. Effect of endogenous selenium on arsenic uptake and antioxidative enzymes in as-exposed rice seedlings. Int. J. Environ. Res. Public Health 2019, 16, 3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Treatment (μM AsV) | TFroot to shoot |
|---|---|
| 0 | - |
| 10 | 0.047 |
| 25 | 0.056 |
| 50 | 0.085 |
| 100 | 0.096 |
| 200 | 0.197 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solórzano, E.; Corpas, F.J.; González-Gordo, S.; Palma, J.M. Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress. Agronomy 2020, 10, 1014. https://doi.org/10.3390/agronomy10071014
Solórzano E, Corpas FJ, González-Gordo S, Palma JM. Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress. Agronomy. 2020; 10(7):1014. https://doi.org/10.3390/agronomy10071014
Chicago/Turabian StyleSolórzano, Ernestina, Francisco J. Corpas, Salvador González-Gordo, and José M. Palma. 2020. "Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress" Agronomy 10, no. 7: 1014. https://doi.org/10.3390/agronomy10071014
APA StyleSolórzano, E., Corpas, F. J., González-Gordo, S., & Palma, J. M. (2020). Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress. Agronomy, 10(7), 1014. https://doi.org/10.3390/agronomy10071014