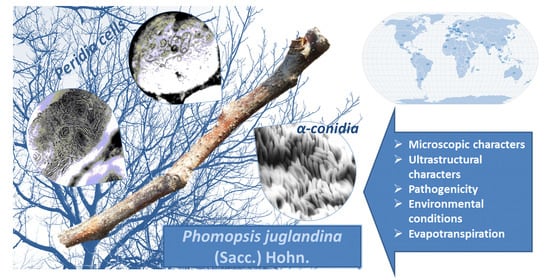
Characteristics of Phomopsis juglandina (Sacc.) Hohn. Associated with Dieback of Walnut in the Climatic Conditions of Southern Romania
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Pathogenicity
3.2. Cultural Characteristics
3.3. Ultrastructural Characteristics
3.3.1. Ultrastructure of the Vegetative Hyphae Cells
3.3.2. Ultrastructure of the Peridia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botu, I.; Botu, M.; Achim, G. Cultura Nucului in Exploataţii Nucicole Moderne; Phoenix: Bucharest, Romania, 2001; p. 174. ISBN 973-85474-2-3. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 1 November 2020).
- Kirk, P.M.; Cannon, P.F.; David, J.C.; Stalpers, J.A. Dictionary of the Fungi, 9th ed.; CAB International: Wallingford, UK, 2001. [Google Scholar]
- Allescher, A. Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz–Fungi Imperfecti; Verlag von Eduard Kummer: Leipzig, Germany, 1900; Volume VI, IX. [Google Scholar]
- Diego, E.L.F.; Santos, J.M.; Phillips, A.J.L. Phylogeny, morphology and pathogenicity of Diaporthe and Phomopsis species on almond in Portugal. Fungal Divers. 2010, 44, 107–115. [Google Scholar] [CrossRef]
- USDA. Index of Plant Diseases in the United States; USDA Agric. Handb.: Washington, DC, USA, 1960; pp. 1–531.
- Benschop, K.; Tewari, J.P.; Toop, E.W. Phomopsis twig die-back of some woody interior ornamentals in Alberta. Can. Plant. Dis. Surv. 1984, 64, 29–31. [Google Scholar]
- Claydon, N.; Grove, J.F.; Pople, M. Elm bark beetle boring and feeding deterrents from Phomopsis oblonga. Phytochemistry 1985, 24, 937–943. [Google Scholar] [CrossRef]
- Farr, D.F.; Castlebury, L.A.; Rossman, A.Y. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States. Mycologia 2002, 94, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, C.C. Bolile Criptogamice din Pepiniere şi Plantaţii; ICES: Bucharest, Romania, 1955; Volume II, p. 6. [Google Scholar]
- Boddy, L.; Griffith, G.S. Role of endophytes and latent invasion in the development of decay communities in sapwood of angiospermous trees. Sydowia 1989, 41, 41–73. [Google Scholar]
- Charudattan, R. Current status of biological control of weeds. In Emerging Technologies for Integrated Pest Management: Concepts, Research, and Implementation; Kennedy, G.G., Sutton, T.B., Eds.; APS: St. Paul, MN, USA, 2000; pp. 269–288. [Google Scholar]
- Bechet, M.; Silaghi, G.; Turcu, L.; Lörinczi, F. Flora micologică. In Flora şi Vegetaţia Rezervaţiei Naturale Defileul Crişului Repede; Contributii Botanice Universitatea Babes-Bolyai din Cluj-Napoca: Cluj-Napoca, Romania, 1966; Volume I, pp. 31–82. [Google Scholar]
- Green, R.J. Dieback of black walnut seedlings caused by Phomopsis elaeagni. Plant. Dis. Rep. 1977, 61, 5825–5884. [Google Scholar]
- Vercesi, A.M. Disseccamenti da Phomopsis su noce. Inf. Fitopatol. 1982, 12, 51–54. [Google Scholar]
- Kanematsu, S.; Kobayashi, T.; Kudo, A.; Ohtsu, Y. Conidial morphology, pathogenicity and culture characteristics of Phomopsis isolates from peach, Japanese pear and apple in Japan. Ann. Phytopathol. Soc. Jpn. 1999, 65, 264–273. [Google Scholar] [CrossRef]
- Mihaescu, C. Ultrastructural aspect of structure somatic and reproductive of the species of Phomopsis (Sacc.) Bubák. Curr. Trends Nat. Sci. 2017, 6, 311–314. [Google Scholar]
- Cristescu, C. The morphology and anatomy of structure somatic and reproductive of species of Phomopsis Sacc. Bubak. Bul. Grădinii Bot. Iaşi. 2007, 14, 19–27. [Google Scholar]
- Sutton, B.C. The Coelomycetes: Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980. [Google Scholar]
- Chen, Y.Q.; Jiang, Z. Application of RAPD and ITS region sequence analyses on classification and identification of Phomopsis. Mycosystema 2002, 21, 39–46. [Google Scholar]
- Luo, L.J.; Xi, P.G.; Jiang, Z.; Qi, P.K. Sporulation conditions of Phomopsis in pure culture. Mycosystema 2004, 23, 219–225. [Google Scholar]
- Uecker, F.A.; Johnson, D.A. Morphology and taxonomy of species of Phomopsis on Asparagus. Mycologia 1991, 83, 192–199. [Google Scholar] [CrossRef]
- Montañola-Cvetković, M.; Bojovic-Cvetic, D.; Vukojevic, J. An ultrastructural study of α and β conidia in the fungal genus. Phomopsis. Cryptogam. Mycol. 1985, 6, 171–184. [Google Scholar]
- Botu, I.; Botu, M. Metode şi Tehnici de Cercetare în Pomicultură; Editura Conphys: Rm. Valcea, Romania, 1997; p. 327. ISBN 973-9334-08-03. [Google Scholar]
- SPSS Tutorials: Home. Available online: https://libguides.library.kent.edu/SPSS/PearsonCorr (accessed on 1 November 2020).
- Bartnicki-Garcia, S. Role of Vesicle in Apical Growth and a New Mathematical Model of Hyphal Morphogenesis; Heath, I.B., Ed.; Academic: San Diego, CA, USA, 1990; pp. 211–232. [Google Scholar]
- Coppin, E.; Debuchy, R.; Arnaise, S.; Picard, M. Mating types and sexual development in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 1997, 61, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.M. Fungal Organelles and other Cell Structures; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Uecker, F.A. A world list of Phomopsis names with notes on nomenclature, morphology and biology. Mycol. Mem. 1988, 13, 1–231. [Google Scholar]
- Guido, M.A.D.; Pollastro, S.; Carlucci, A.; Angelini, R.M.D.M.; Faretra, F. Phomopsis viticola is easily transformed with hph and Bmlr genes. J. Plant. Pathol. 2003, 85, 43–52. [Google Scholar]
- Udayanga, D.; Liu, X.; McKenzie, E.H.C.; Chukeatirote, E.; Bahkali, A.H.A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Simon, F.; Fischl, G.; Kadlicskó, S.; Pintér, C.S.; Dankó, J.; Süle, S. Phytopatological problems and solutions in the walnut orchards along Lake Balaton. Commmun. Agric. Appl. Biol. Sci. 2007, 72, 765–770. [Google Scholar]
- Ágreda, T.; Águeda, B.; Olano, J.M.; Vicente-Serrano, S.M.; Fernández-Toirán, M. Increased evapotranspiration demand in a Mediterranean climate might cause a decline in fungal yields under global warming. Glob. Chang. Biol. 2015, 21, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, W.B. The Fifth Kingdom, 3rd ed.; Focus Publishing, R. Pullins Company Newburyport: Newburyport, MA, USA, 2000. [Google Scholar]
- Hilario, S.; Amaral, I.A.; Goncalves, M.F.M.; Lopes, A.; Santos, L.; Alves, A. Diaporthe species associated with twig blight and dieback of Vaccinium corymbosum in Portugal, with description of four new species. Mycologia 2020, 112, 293–308. [Google Scholar] [CrossRef] [PubMed]
- León, M.; Berbegal, M.; Rodríguez-Reina, J.M.; Elena, G.; Abad-Campos, P.; Ramón-Albalat, A.; Olmo, D.; Vicent, A.; Luque, J.; Miarnau, X.; et al. Identification and Characterization of Diaporthe spp. Associated with Twig Cankers and Shoot Blight of Almonds in Spain. Agronomy 2020, 10, 1062. [Google Scholar] [CrossRef]
- Eichmeier, A.; Pecenka, J.; Spetik, M.; Necas, T.; Ondrasek, I.; Armengol, J.; León, M.; Berlanas, C.; Gramaje, D. Fungal Trunk Pathogens Associated with Juglans regia in the Czech Republic. Plant. Dis. 2020, 104, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Dunea, D. SBC-MEDIU: A multi-expert system for environmental diagnosis. Environ. Eng. Manag. J. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Michailides, T.J. Botryopshaeria, Phomopsis and Anthracnose Management in Walnuts; Quad-County Walnut: Davis, UC, USA, 2019. [Google Scholar]
- Dunea, D.; Dincă, N. Improving land utilization using intensive grass-clover mixtures in forage production systems. Rom. Agric. Res. 2014, 31, 147–158. [Google Scholar]
- Sittisart, P.; Yossan, S.; Prasertsan, P. Antifungal property of chili, shallot and garlic extracts against pathogenic fungi, Phomopsis spp., isolated from infected leaves of para rubber (Hevea brasiliensis Muell. Arg.). Agric. Nat. Resour. 2017, 51, 485–491. [Google Scholar] [CrossRef]
- Wessels, J.G.H. Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkorper, cytoskeleton and endomembranes after freeze-substitution. J. Cell Sci. 1981, 48, 89–103. [Google Scholar]
- Howard, R.J. Wall growth, protein excretion and morphogenesis in fungi. New Phytol. 1993, 3, 397–413. [Google Scholar]
- Vargas, M.; Aronson, J.M.; Roberson, R.W. The cytoplasmic organization of hyphal tip cell in the fungus Allomyces macrogynus. Protoplasma 1993, 176, 43–52. [Google Scholar] [CrossRef]
- Cole, L.; Orlovich, D.; Ashfors, A.E. Structure, function and motility of vacuoles in Filamentous Fungi. Fungal Genet. Biol. 1998, 24, 86–100. [Google Scholar] [CrossRef]
- Lousa, C.M.; Denecke, J. Lysosomal and vacuolar sorting: Not so different after all! Biochem. Soc. Trans. 2016, 44, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, H.B.; Greenwood, A. The concentric bodies of lichenized fungi. Biology 2004, 87, 285–302. [Google Scholar] [CrossRef]









| ETo | P (mm) | T (°C) | Exc (mm) | Def (mm) | LL1Y | LL2Y | |
|---|---|---|---|---|---|---|---|
| ETo | 1 | 0.503 | 0.949 ** | 0.086 | 0.530 | −0.986 ** | −0.962 ** |
| - | 0.250 | 0.001 | 0.854 | 0.221 | 0.000 | 0.001 | |
| P (mm) | - | 1 | 0.390 | 0.807 * | −0.411 | −0.617 | −0.622 |
| - | - | 0.387 | 0.028 | 0.360 | 0.140 | 0.136 | |
| T (°C) | - | - | 1 | 0.038 | 0.613 | −0.909 ** | −0.870 * |
| - | - | - | 0.936 | 0.144 | 0.005 | 0.011 | |
| Exc (mm) | - | - | - | 1 | −0.499 | −0.229 | −0.284 |
| - | - | - | - | 0.254 | 0.621 | 0.538 | |
| Def (mm) | - | - | - | - | 1 | −0.427 | −0.428 |
| - | - | - | - | - | 0.339 | 0.338 | |
| LL1Y | - | - | - | - | - | 1 | 0.988 ** |
| - | - | - | - | - | - | 0.000 | |
| LL2Y | - | - | - | - | - | - | 1 |
| - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaescu, C.; Dunea, D.; Bășa, A.G.; Frasin, L.N. Characteristics of Phomopsis juglandina (Sacc.) Hohn. Associated with Dieback of Walnut in the Climatic Conditions of Southern Romania. Agronomy 2021, 11, 46. https://doi.org/10.3390/agronomy11010046
Mihaescu C, Dunea D, Bășa AG, Frasin LN. Characteristics of Phomopsis juglandina (Sacc.) Hohn. Associated with Dieback of Walnut in the Climatic Conditions of Southern Romania. Agronomy. 2021; 11(1):46. https://doi.org/10.3390/agronomy11010046
Chicago/Turabian StyleMihaescu, Cristina, Daniel Dunea, Adrian Gheorghe Bășa, and Loredana Neagu Frasin. 2021. "Characteristics of Phomopsis juglandina (Sacc.) Hohn. Associated with Dieback of Walnut in the Climatic Conditions of Southern Romania" Agronomy 11, no. 1: 46. https://doi.org/10.3390/agronomy11010046
APA StyleMihaescu, C., Dunea, D., Bășa, A. G., & Frasin, L. N. (2021). Characteristics of Phomopsis juglandina (Sacc.) Hohn. Associated with Dieback of Walnut in the Climatic Conditions of Southern Romania. Agronomy, 11(1), 46. https://doi.org/10.3390/agronomy11010046