Canopy Spraying of Abscisic Acid to Improve Fruit Quality of Different Sweet Cherry Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Exogenous Application of ABA and NDGA
2.2. Sample Collection and Fruit Quality Parameters Analysis
3. Results
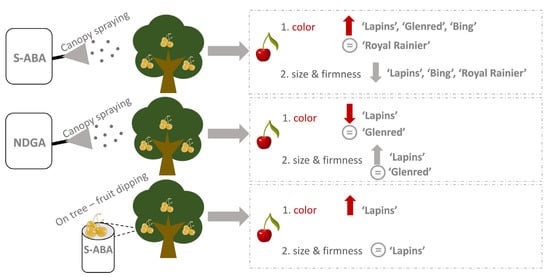
3.1. The Effect of the ABA Canopy Spraying on Sweet Cherry Fruit Quality Parameters of the ‘Glenred’, ‘Lapins’, ‘Bing’ and ‘Royal Rainier’ Cultivars during the 2017 Season
3.2. The Effect of ABA and NDGA Canopy Spraying on Sweet Cherry Fruit Quality Parameters of Cultivars ‘Glenred’ and ‘Lapins’ during the 2018 Season
3.3. The Effect of “On-Tree” ABA Fruit Dipping on Sweet Cherry Fruit Quality Parameters of Cultivar ‘Lapins’
4. Discussion
4.1. Cultivar Dependent Effect of ABA Canopy Spraying of Sweet Cherry Trees on Fruit Quality Parameters
4.2. ABA Effect Is Different between Canopy Spraying and Dipping Treatment
5. Conclusions
- ABA canopy spraying of sweet cherry trees alters fruit quality parameters in a cultivar dependent manner;
- Reproducible variations in sweet cherry fruit quality parameters in ABA canopy sprayed ‘Lapins’ cultivar trees were detected in consecutive seasons (improving fruit color but reducing fruit size and firmness);
- Canopy spraying of sweet cherry trees with the ABA biosynthesis inhibitor, NDGA, altered fruit color and size in the mid/late-maturing cultivar (‘Lapins’), but not the early-maturing cultivar (Glen Red);
- Variable seasonal responses of the early-maturing cultivar (‘Glenred’) to ABA canopy spraying suggests that the ABA response in this cultivar may be influenced by environmental factors. Further investigation is needed to identify these environmental factors and how these factors influence the response to ABA canopy spraying in the fruits of this cultivar;
- ABA “on-tree” fruit dipping treatment in the ‘Lapins’ cultivar improves sweet cherry fruit color without negatively affecting fruit size, whereas ABA canopy spraying improves fruit color but decreases fruit size. These finding suggests that the decrease in fruit size in response to the ABA canopy spraying may be due to the effect of ABA on the tree foliage.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- iQonsulting. Anuario Cereza 2020–2021. Available online: http://www.iqonsulting.com/yb/ (accessed on 27 September 2021).
- Redagricola.com. Available online: https://www.redagricola.com/cl/assets/uploads/2020/12/ra116.pdf (accessed on 27 September 2021).
- Worldstopexports.com. Available online: https://www.worldstopexports.com/natural-honey-exporters/ (accessed on 27 September 2021).
- Growingproduce.com. Available online: https://www.growingproduce.com/author/glang/ (accessed on 27 September 2021).
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit Development and Ripening. Annu. Rev. Plant Biol. 2013. [Google Scholar] [CrossRef] [Green Version]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A Dynamic Interplay between Phytohormones Is Required for Fruit Development, Maturation, and Ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Cherian, S.; Figueroa, C.R.; Nair, H. “Movers and Shakers” in the Regulation of Fruit Ripening: A Cross-Dissection of Climacteric versus Non-Climacteric Fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jia, H.; Chai, Y.; Shen, Y. Abscisic Acid Perception and Signaling Transduction in Strawberry: A Model for Non-Climacteric Fruit Ripening. Plant Signal. Behav. 2011, 6, 1950–1953. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Jiu, S.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.; Wang, B.; Cui, L.; Fang, J. Abscisic Acid and Sucrose Regulate Tomato and Strawberry Fruit Ripening through the Abscisic Acid-Stress-Ripening Transcription Factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef]
- Kondo, S.; Gemma, H. Relationship between Abscisic Acid (ABA) Content and Maturation of the Sweet Cherry. Engei Gakkai Zasshi 1993, 62, 63–68. [Google Scholar] [CrossRef]
- Kondo, S.; Inoue, K. Abscisic Acid (ABA) and 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Content during Growth of “Satohnishiki” Cherry Fruit, and the Effect of ABA and Ethephon Application on Fruit Quality. J. Hortic. Sci. 1997, 72, 221–227. [Google Scholar] [CrossRef]
- Ren, J.; Chen, P.; Dai, S.; Li, P.; Li, Q.; Ji, K.; Wang, Y.; Leng, P. Role of Abscisic Acid and Ethylene in Sweet Cherry Fruit Maturation: Molecular Aspects. N. Z. J. Crop Hortic. Sci. 2011, 39, 161–174. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.J.; Ren, J.; Zhang, C.X.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q.; et al. The Role of ABA in the Maturation and Postharvest Life of a Nonclimacteric Sweet Cherry Fruit. J. Plant Growth Regul. 2014. [Google Scholar] [CrossRef]
- Kuhn, N.; Ponce, C.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Carrera, E.; Sagredo, B.; Donoso, J.M.; Meisel, L.A. ABA Influences Color Initiation Timing in P. Avium L. Fruits by Sequentially Modulating the Transcript Levels of ABA and Anthocyanin-Related Genes. Tree Genet. Genomes 2021, 17. [Google Scholar] [CrossRef]
- Kuhn, N.; Ponce, C.; Arellano, M.; Time, A.; Sagredo, B.; Donoso, J.M.; Meisel, L.A. Gibberellic Acid Modifies the Transcript Abundance of ABA Pathway Orthologs and Modulates Sweet Cherry (Prunus Avium) Fruit Ripening in Early- and Mid-Season Varieties. Plants 2020, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of Abscisic Acid and Methyl Jasmonate Preharvest Applications on Fruit Quality and Cracking Tolerance of Sweet Cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Lemus, G. El Cultivo Del Cerezo. In Boletín INIA N° 133; Primera Edición; 2005; p. 255. [Google Scholar]
- Gonzalez, C. Efecto de Diferentes Portainjertos de Cerezo Sobre El Comportamiento Fenológico de Los Cultivares Lapins, Bing y Sweetheart, En San Francisco de Mostazal (VI Región). Tesis de Maestría, Pontifica Universidad Católica de Valapraiso, Quillota, Chile, 25 May 2004. [Google Scholar]
- Chavoshi, M.; Watkins, C.; Oraguzie, B.; Zhao, Y.; Iezzoni, A.; Oraguzie, N. Phenotyping Protocol for Sweet Cherry (Prunus Avium L.) to Facilitate an Understanding of Trait Inheritance. J. Am. Pomol. Soc. 2014, 68, 125–134. [Google Scholar]
- Teribia, N.; Tijero, V.; Munné-Bosch, S. Linking Hormonal Profiles with Variations in Sugar and Anthocyanin Contents during the Natural Development and Ripening of Sweet Cherries. New Biotechnol. 2016, 33, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Kappel, F.; MacDonald, R. Early Gibberellic Acid Sprays Increase Firmness and Fruit Size of “Sweetheart” Sweet Cherry. J. Am. Pomol. Soc. 2007, 61, 38. [Google Scholar]
- Choi, C.; Toivonen, P.; Wiersma, P.A.; Kappel, F. Effect of Gibberellic Acid during Development of Sweet Cherry Fruit: Physiological and Molecular Changes. Acta Hortic. 2004, 636, 489–495. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Štampar, F. Physicochemical Changes of Sweet Cherry Fruits Related to Application of Gibberellic Acid. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
- Zilkah, S.; David, I.; Rotbaum, A.; Faingersh, E.; Lurie, S.; Weksler, A. Effect of Plant Growth Regulators on Extending the Marketing Season of Sweet Cherry. Acta Hortic. 2008, 795, 699–702. [Google Scholar] [CrossRef]
- Yildirim, A.N.; Koyuncu, F. The Effect of Gibberellic Acid Applications on the Cracking Rate and Fruit Quality in the “0900 Ziraat” Sweet Cherry Cultivar. African J. Biotechnol. 2010, 9. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M. Plant Growth Regulators Improve Sweet Cherry Fruit Quality without Reducing Endocarp Growth. Sci. Hortic. 2013, 150, 73–79. [Google Scholar] [CrossRef]
- Kuhn, N.; Maldonado, J.; Ponce, C.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Carrera, E.; Donoso, J.M.; Sagredo, B.; et al. RNAseq Reveals Different Transcriptomic Responses to GA3 in Early and Midseason Varieties before Ripening Initiation in Sweet Cherry Fruits. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.; Lauvergeat, V.; Gomès, E.; Li, S.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry Ripening: Recently Heard through the Grapevine. J. Exp. Bot. 2013, 65, 4543–4559. [Google Scholar] [CrossRef] [Green Version]
- Nagpala, E.G.L.; Noferini, M.; Farneti, B.; Piccinini, L.; Costa, G. Cherry-Meter: An Innovative Non-Destructive (Vis/NIR) Device for Cherry Fruit Ripening and Quality Assessment. Acta Hortic. 2017, 1161, 491–496. [Google Scholar] [CrossRef]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The Relationship between the Expression of Abscisic Acid Biosynthesis Genes, Accumulation of Abscisic Acid and the Promotion of Vitis Vinifera L. Berry Ripening by Abscisic Acid. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The Biosynthesis of Flavonoids Is Enhanced Similarly by UV Radiation and Root Zone Salinity in L. Vulgare Leaves. J. Plant Physiol. 2011, 168. [Google Scholar] [CrossRef] [PubMed]
- Ponce, C.; Kuhn, N.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Carrera, E.; Sagredo, B.; Donoso, J.M.; Meisel, L.A. Differential Phenolic Compounds and Hormone Accumulation Patterns between Early- and Mid-Maturing Sweet Cherry (Prunus Avium L.) Cultivars during Fruit Development and Ripening. J. Agric. Food Chem. 2021, 69, 8850–8860. [Google Scholar] [CrossRef] [PubMed]
- Quero-García, J.; Campoy, J.A.; Castède, S.; Pitiot, C.; Barreneche, T.; Lerigoleur-Balsemin, E.; Wenden, B.; Le Dantec, L.; Dirlewanger, E. Breeding Sweet Cherries at INRA-Bordeaux: From Conventional Techniques to Marker-Assisted Selection. Acta Hortic. 2017, 1161, 1–13. [Google Scholar] [CrossRef]
- Guajardo, V.; Solís, S.; Gasic, K.; Saski, C.; Font i Forcada, C.; Moreno, M. Genotyping-by-Sequencing (GBS) for SNP-Based Linkage Map Construction for Two Prunus Rootstocks from a Peach Rootstock Breeding Program. Acta Hortic. 2021, 1304, 113–120. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Li, M.; Wang, J.; Yang, Y.; Zhang, X.; Yan, G.; Zhang, H.; Liu, J.; Zhang, K. The R2R3 MYB Transcription Factor PavMYB10.1 Involves in Anthocyanin Biosynthesis and Determines Fruit Skin Colour in Sweet Cherry (Prunus Avium L.). Plant Biotechnol. J. 2016, 14, 2120–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; García, K.; Méndez, M.A.; González, M.; Meisel, L.A.; Defilippi, B.G.; del Pozo, T. Melatonin Triggers Metabolic and Gene Expression Changes Leading to Improved Quality Traits of Two Sweet Cherry Cultivars during Cold Storage. Food Chem. 2020, 319. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Pacifico, A.; Gambacorta, G.; Faccia, M.; Trani, A.; Gallo, V.; Cafagna, I.; et al. Application of Abscisic Acid (S-ABA) to “Crimson Seedless” Grape Berries in a Mediterranean Climate: Effects on Color, Chemical Characteristics, Metabolic Profile, and S-ABA Concentration. J. Plant Growth Regul. 2013, 32, 491–505. [Google Scholar] [CrossRef]
- Koyama, R.; Colombo, R.C.; Silva Borges, W.F.; Silvestre, J.P.; Hussain, I.; Shahab, M.; Ahmed, S.; Prudencio, S.H.; Teodoro de Souza, R.; Roberto, S.R. Abscisic Acid Application Affects Color and Acceptance of the New Hybrid ‘BRS Melodia’ Seedless Grape Grown in a Subtropical Region. HortScience 2019, 54, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Coombe, B.G.; Hale, C.R. The Hormone Content of Ripening Grape Berries and the Effects of Growth Substance Treatments. Plant Physiol. 1973, 51, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of Exogenous Abscisic Acid on Antioxidant Capacities, Anthocyanins, and Flavonol Contents of Muscadine Grape (Vitis Rotundifolia) Skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Kretzschmar, A.A.; Lerin, S.; Fagherazzi, A.F.; Mario, A.E.; Bastos, F.E.A.; Allebrandt, R.; Rufato, L. Application of Abscisic Acid Increases the Colour of “Rubi” Grape Berries in Southern Brazil. Acta Hortic. 2016, 1115, 231–236. [Google Scholar] [CrossRef]
- Leão, P.C.d.S.; Lima, M.A.C.; Costa, J.P.D.; da Trindade, D.C.G. Abscisic Acid and Ethephon for Improving Red Color and Quality of Crimson Seedless Grapes Grown in a Tropical Region. Am. J. Enol. Vitic. 2015, 66, 37–45. [Google Scholar] [CrossRef]
- Rodrigo, J.; Guerra, M.E. Cerezo y Ciruelo. In La Fruticultura. del Siglo XXI en España, l; Hueso Martin, J.J., Cuevas González, J., Eds.; Cajamar Caja Rural: Madrid, Spain, 2014; pp. 107–122. [Google Scholar] [CrossRef]










| Statistical Variations in the Physiological Parameters of Sweet Cherry Fruits Post-Treatment at Harvest | ||||||||
|---|---|---|---|---|---|---|---|---|
| Season-Treatment | Cultivar | Width | Weight | Firmness | IAD | TSSC | TA | TSSC/TA |
| 2017—ABA canopy spraying | ‘Glenred’ | ↑ | ↑ | ↓ | - | - | - | - |
| ‘Lapins’ | ↓ | ↓ | ↓ | ↑ | - | - | - | |
| ‘Bing’ | ↓ | ↓ | ↓ | ↑ | ↑ | - | - | |
| ‘R. Rainier’ | ↓ | ↓ | ↑ | - | - | - | - | |
| 2018—ABA canopy spraying | ‘Glenred’ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ |
| ‘Lapins’ | ↓ | ↓ | - | ↑ | - | - | - | |
| 2018—NDGA canopy spraying | ‘Glenred’ | - | - | ↓ | - | - | - | - |
| ‘Lapins’ | ↓ | - | - | - | - | - | - | |
| 2018—ABA fruit dipping | ‘Lapins’ | - | - | ↓ | ↑ | - | - | - |
| 2019—ABA fruit dipping | ‘Lapins’ | - | - | - | ↑ | ↑ | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Time, A.; Ponce, C.; Kuhn, N.; Arellano, M.; Sagredo, B.; Donoso, J.M.; Meisel, L.A. Canopy Spraying of Abscisic Acid to Improve Fruit Quality of Different Sweet Cherry Cultivars. Agronomy 2021, 11, 1947. https://doi.org/10.3390/agronomy11101947
Time A, Ponce C, Kuhn N, Arellano M, Sagredo B, Donoso JM, Meisel LA. Canopy Spraying of Abscisic Acid to Improve Fruit Quality of Different Sweet Cherry Cultivars. Agronomy. 2021; 11(10):1947. https://doi.org/10.3390/agronomy11101947
Chicago/Turabian StyleTime, Alson, Claudio Ponce, Nathalie Kuhn, Macarena Arellano, Boris Sagredo, José Manuel Donoso, and Lee A. Meisel. 2021. "Canopy Spraying of Abscisic Acid to Improve Fruit Quality of Different Sweet Cherry Cultivars" Agronomy 11, no. 10: 1947. https://doi.org/10.3390/agronomy11101947
APA StyleTime, A., Ponce, C., Kuhn, N., Arellano, M., Sagredo, B., Donoso, J. M., & Meisel, L. A. (2021). Canopy Spraying of Abscisic Acid to Improve Fruit Quality of Different Sweet Cherry Cultivars. Agronomy, 11(10), 1947. https://doi.org/10.3390/agronomy11101947