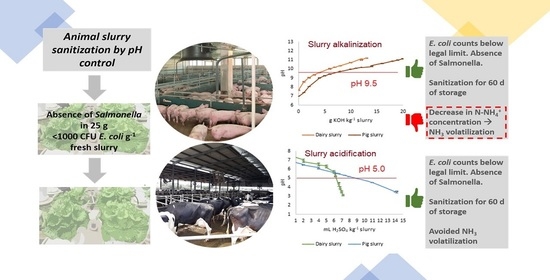
Animal Slurry Sanitization through pH Adjustment: Process Optimization and Impact on Slurry Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Dairy and Pig Slurries
2.2. Physicochemical Analysis of the Slurries
2.3. Microbiological Analysis of the Slurries
2.3.1. Detection of Salmonella
2.3.2. Enumeration of Escherichia coli
2.4. Slurry Alkalinization Tests
2.5. Slurry Acidification Tests
2.6. Storage Experiment Setup
2.7. Statistical Analysis of the Data
3. Results and Discussion
3.1. Slurry Sanitization by pH Modification
3.1.1. Alkalinization
3.1.2. Acidification
3.1.3. Slurry Characteristics That Affect pH Adjustment and Sanitization
3.2. Influence of Storage Time on the Sanitization of Raw and Treated AS
3.3. Influence of the Treatment and Storage Time on the Physicochemical Characteristics of Raw and Treated AS
3.4. Practical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agricultural Production—Livestock and Meat—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Agricultural_production_-_livestock_and_meat (accessed on 5 February 2021).
- FAO (Ed.) Livestock in the Balance; The State of Food and Agriculture; FAO: Rome, Italy, 2009; ISBN 978-92-5-106215-9. [Google Scholar]
- Bittman, S.; Dedina, M.; Howard, C.M.; Oenema, O.; Sutton, M.A. Options for Ammonia Mitigation: Guidance from the UNECE Task Force on Reactive Nitrogen; Centre for Ecology & Hydrology, on Behalf of Task Force on Reactive Nitrogen, of the UNECE Convention on Long Range transboundary Air Pollution: Edinburgh, UK, 2014; ISBN 978-1-906698-46-1. [Google Scholar]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure Management: Implications for Greenhouse Gas Emissions. Anim. Feed Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; de Vries, W.; Weiss, F.; et al. Impacts of European Livestock Production: Nitrogen, Sulphur, Phosphorus and Greenhouse Gas Emissions, Land-Use, Water Eutrophication and Biodiversity. Environ. Res. Lett. 2015, 10, 115004. [Google Scholar] [CrossRef]
- Parker, D.B.; Gilley, J.; Woodbury, B.; Kim, K.-H.; Galvin, G.; Bartelt-Hunt, S.L.; Li, X.; Snow, D.D. Odorous VOC Emission Following Land Application of Swine Manure Slurry. Atmos. Environ. 2013, 66, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/1009, of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. OJ 170. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 5 February 2021).
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to Reduce Pathogen Microorganisms in Manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Skowron, K.; Olszewska, H.; Skowron, K.; Paluszak, P.; Breza-Boruta, B. Hygienic Aspect of Cattle Slurry Storage as Most Popular and Cheapest Method of Handling Liquid Animal Excrements. Ann. Anim. Sci. 2013, 13, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Overview of Hygienization Pretreatment for Pasteurization and Methane Potential Enhancement of Biowaste: Challenges, State of the Art and Alternative Technologies. J. Clean. Prod. 2019, 236, 117525. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of Animal Slurry—A Review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Surgy, S.; Napier, V.; Menaia, J.; Vasconcelos, E.; Coutinho, J. Impact of Slurry Management Strategies on Potential Leaching of Nutrients and Pathogens in a Sandy Soil Amended with Cattle Slurry. J. Environ. Manag. 2014, 146, 198–205. [Google Scholar] [CrossRef]
- Soares, A.S.; Miranda, C.; Teixeira, C.A.; Coutinho, J.; Trindade, H.; Coelho, A.C. Impact of Different Treatments on Escherichia Coli during Storage of Cattle Slurry. J. Environ. Manag. 2019, 236, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Bina, B.; Movahedian, H.; Kord, I. The Effect of Lime Stabilization on the Microbiological Quality of Sewage. Sludge 2004, 5, 34–38. [Google Scholar]
- Anderson, C.; Malambo, D.H.; Gonzalez Perez, M.E.; Nobela, H.N.; de Pooter, L.; Spit, J.; Hooijmans, C.M.; van de Vossenberg, J.; Greya, W.; Thole, B.; et al. Lactic Acid Fermentation, Urea and Lime Addition: Promising Faecal Sludge Sanitizing Methods for Emergency Sanitation. Int. J. Environ. Res. Public Health 2015, 12, 13871–13885. [Google Scholar] [CrossRef] [Green Version]
- Arthurson, V. Proper Sanitization of Sewage Sludge: A Critical Issue for a Sustainable Society. Appl. Environ. Microbiol. 2008, 74, 5267–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strande, L. Faecal Sludge Management—Systems Approach for Implementation and Operation; IWA Publishing: London, UK, 2014; Volume 13. [Google Scholar] [CrossRef] [Green Version]
- Farzadkia, M.; Bazrafshan, E. Lime Stabilization of Waste Activated Sludge. Health Scope 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Bolton, D.J.; Ivory, C.; McDowell, D.A. The Effect of Urea and Ammonia Treatments on the Survival of Salmonella Spp. and Yersinia Enterocolitica in Pig Slurry. J. Appl. Microbiol. 2013, 114, 134–140. [Google Scholar] [CrossRef]
- Ottoson, J.; Nordin, A.; von Rosen, D.; Vinnerås, B. Salmonella Reduction in Manure by the Addition of Urea and Ammonia. Bioresour. Technol. 2008, 99, 1610–1615. [Google Scholar] [CrossRef]
- Nour, A.; Alsewailem, M.; El-Naggar, A. The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents. Sustainability 2019, 11, 2441. [Google Scholar] [CrossRef] [Green Version]
- Santos, J. Fertilização—Fundamentos Agroambientais da Utilização dos Adubos e Correctivos; Publindústria: Porto, Portugal, 2015; ISBN 978-989-723-085-1. [Google Scholar]
- Broadbent, F.E. The Soil Organic Fraction. In Advances in Agronomy; Norman, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1953; Volume 5, pp. 153–183. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Critl. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- ISO 6579-1:2017, Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/67/56712.html (accessed on 25 June 2020).
- ISO 16649-2:2015. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of 2-Glucoronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 Degrees Using 5-Bromo-4-Chloro-3-Indolyl 2-D-Glucuronide. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/68/56824.html (accessed on 25 June 2020).
- Negri, M.; Calcaterra, C.; Rizzi, M.; Scevola, M.E. Influenza Del PH Sulla Carica Batterica Nei Fanghi Di Depurazione. In Acque Reflue e Fanghi; A. Frigerio, Centro Scientifico Internazionale: Milan, Italy, 1991; pp. 260B–272B. [Google Scholar]
- Strauch, D. Pathogenic Microrganisms in Sludge—AnaerobicDigestion and Disinfection Methods to Make Sludge Usable as Fertilizer. Eur. Water Manag. 1998, 1, 12–26. [Google Scholar]
- Chembid—Find and Compare Offers for Chemicals. Available online: https://www.chembid.com/en/ (accessed on 9 February 2021).
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Sparks, D.L. 10—The Chemistry of Saline and Sodic Soils. In Environmental Soil Chemistry, 2nd ed.; Sparks, D.L., Ed.; Academic Press: Burlington, NY, USA, 2003; pp. 285–300. ISBN 978-0-12-656446-4. [Google Scholar] [CrossRef]
- Council Directive 91/676/EEC Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. 1991, pp. 1–8. Available online: https://eur-lex.europa.eu/eli/dir/1991/676/oj (accessed on 4 February 2021).
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to Sulfuric Acid for Slurry Acidification: Impact on Slurry Composition and Ammonia Emissions during Storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- D’Annibale, A.; Labouriau, R.; Sørensen, P.; Krogh, P.H.; Christensen, B.T.; Eriksen, J. Effect of Acidified Cattle Slurry on a Soil Collembolan Community: A Mesocosmos Study. Eur. J. Soil Biol. 2019, 94, 103117. [Google Scholar] [CrossRef]
- Edesi, L.; Talve, T.; Akk, E.; Võsa, T.; Saue, T.; Loide, V.; Vettik, R.; Plakk, T.; Tamm, K. Effects of Acidified Pig Slurry Application on Soil Chemical and Microbiological Properties under Field Trial Conditions. Soil Tillage Res. 2020, 202, 104650. [Google Scholar] [CrossRef]
- Fangueiro, D.; Pereira, J.L.S.; Fraga, I.; Surgy, S.; Vasconcelos, E.; Coutinho, J. Band Application of Acidified Slurry as an Alternative to Slurry Injection in a Mediterranean Double Cropping System: Agronomic Effect and Gaseous Emissions. Agric. Ecosyst. Environ. 2018, 267, 87–99. [Google Scholar] [CrossRef]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of Ammonia and Greenhouse Gas Emissions from Stored Cattle Slurry Using Acidifiers and Chemical Amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Christensen, M.L.; Sommer, S.G. Manure Characterisation and Inorganic Chemistry. In Animal Manure Recycling; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013; pp. 41–65. ISBN 978-1-118-67667-7. [Google Scholar]
- Fangueiro, D.; Gusmão, M.; Grilo, J.; Porfírio, G.; Vasconcelos, E.; Cabral, F. Proportion, Composition and Potential N Mineralisation of Particle Size Fractions Obtained by Mechanical Separation of Animal Slurry. Biosyst. Eng. 2010, 106, 333–337. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Sant’Ana, A.S. Manure-Borne Pathogens as an Important Source of Water Contamination: An Update on the Dynamics of Pathogen Survival/Transport as Well as Practical Risk Mitigation Strategies. Int. J. Hyg. Environ. Health 2020, 227, 113524. [Google Scholar] [CrossRef] [PubMed]
- Mohaibes, M.; Heinonen-Tanski, H. Aerobic Thermophilic Treatment of Farm Slurry and Food Wastes. Bioresour. Technol. 2004, 95, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, T.; Doyle, M.P. Fate of Enterohemorrhagic Escherichia Coli O157:H7 in Bovine Feces. Appl. Environ. Microbiol. 1996, 62, 2567–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, J.; Sørensen, P.; Elsgaard, L. The Fate of Sulfate in Acidified Pig Slurry during Storage and Following Application to Cropped Soil. J. Environ. Qual. 2008, 37, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Fangueiro, D.; Ribeiro, H.; Vasconcelos, E.; Coutinho, J.; Cabral, F. Treatment by Acidification Followed by Solid–Liquid Separation Affects Slurry and Slurry Fractions Composition and Their Potential of N Mineralization. Bioresour. Technol. 2009, 100, 4914–4917. [Google Scholar] [CrossRef]
- Kai, P.; Pedersen, P.; Jensen, J.E.; Hansen, M.N.; Sommer, S.G. A Whole-Farm Assessment of the Efficacy of Slurry Acidification in Reducing Ammonia Emissions. Eur. J. Agron. 2008, 28, 148–154. [Google Scholar] [CrossRef]
- Petersen, S.O.; Andersen, A.J.; Eriksen, J. Effects of Cattle Slurry Acidification on Ammonia and Methane Evolution during Storage. J. Environ. Qual. 2012, 41, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Grattan, S.R.; Grieve, C.M. Salinity–Mineral Nutrient Relations in Horticultural Crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R.E. A Review of Ammonia Emission Mitigation Techniques for Concentrated Animal Feeding Operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.H.; Joo, D.J.; Choi, S.J.; Ha, T.W.; Lee, D.H.; Park, I.H.; Jeong, Y.S. Removal of Ammonia by Biofilters: A Study with Flow-Modified System and Kinetics. J. Air Waste Manag. Assoc. 2003, 53, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.L.S.; Perdigão, A.; Marques, F.; Coelho, C.; Mota, M.; Fangueiro, D. Evaluation of Tomato-Based Packing Material for Retention of Ammonia, Nitrous Oxide, Carbon Dioxide and Methane in Gas Phase Biofilters: A Laboratory Study. Agronomy 2021, 11, 360. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Ying, H.; Luo, H. Effects of Acidification during Storage on Emissions of Methane, Ammonia, and Hydrogen Sulfide from Digested Pig Slurry. Biosyst. Eng. 2014, 122, 23–30. [Google Scholar] [CrossRef]
- Jacobsen, B. Costs of Slurry Separation Technologies and Alternative Use of Solid Fraction for Biogas and Burning. Int. J. Agric. Manag. 2011, 1, 11–22. [Google Scholar]

| Slurry Sample ID | DS1 | DS2 | PS1 | PS2 | PS3 | ||
|---|---|---|---|---|---|---|---|
| Sampling Date | 12 March 2019 | 21 May 2019 | 12 February 2019 | 13 March 2019 | 21 May 2019 | ||
| Physicochemical parameters | pH | 7.49 | 7.13 | 7.05 | 7.21 | 6.69 | |
| EC | mS cm−1 | 10.17 | 14.13 | 24.27 | 26.30 | 22.77 | |
| DM | g kg−1 | 93.8 | 97.8 | 87.9 | 39.1 | 124.1 | |
| C | g kg−1 DM | 409.1 | 421.4 | 457.2 | 393.1 | 465.6 | |
| NT | 33.7 | 36.1 | 68.9 | 116.8 | 49.6 | ||
| N-NH4+ | 12.7 | 16.9 | 44.9 | 82.9 | 29.9 | ||
| P | 8.7 | 7.2 | 15.7 | 20.6 | 13.0 | ||
| K | 28.5 | 27.4 | 33.9 | 59.4 | 31.6 | ||
| Na | 11.0 | 8.4 | 13.9 | 27.8 | 11.6 | ||
| Mg | 7.3 | 5.8 | 9.2 | 12.3 | 10.2 | ||
| Ca | 18.7 | 14.0 | 19.5 | 25.9 | 21.0 | ||
| S | 4.4 | 3.8 | 9.0 | 13.1 | 7.8 | ||
| Cu | mg kg−1 DM | 43 | 38 | 150 | 181 | 159 | |
| Zn | 267 | 219 | 583 | 548 | 674 | ||
| Fe | 2332 | 1779 | 1258 | 1228 | 1912 | ||
| Mn | 314 | 220 | 506 | 619 | 545 | ||
| B | 22 | 27 | 39 | 62 | 56 | ||
| Microbiological parameters | E. coli | CFU g−1 | 6.1(±0.0) × 103 | 3.2(±1.7) × 103 | 3.7(±1.3) × 105 | 1.7(±0.4) × 105 | 5.9(±7.5) × 105 |
| Salmonella | detection in 25 g | Absent | |||||
| Alkaline Additives | Target pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 9.00 | 10.00 | 11.00 | ||||||
| DS | PS | DS | PS | DS | PS | |||
| Hydroxide salts | KOH (P) | pH obtained | 9.06 | 9.00 | 10.04 | 10.08 | 11.11 | 11.00 |
| Dose (g kg−1) | 2.0 | 4.0 | 6.0 | 11.0 | 12.0 | 19.0 | ||
| Ca(OH)2 (P) | pH obtained | 9.05 | 9.09 | 10.13 | 10.09 | 11.26 | 11.07 | |
| Dose (g kg−1) | 4.0 | 6.0 | 8.0 | 11.0 | 11.0 | 16.0 | ||
| NaOH (P) | pH obtained | 8.98 | 9.05 | 9.97 | 10.02 | 11.15 | 11.00 | |
| Dose (g kg−1) | 2.0 | 3.0 | 5.0 | 7.0 | 10.0 | 12.0 | ||
| N-based compounds | Urea (P) | pH obtained | 9.16 | 8.99 | (*) | |||
| Dose (g kg−1) | 2.5 | 5.0 | ||||||
| NH3 (S) | pH obtained | 9.16 | 9.16 | 10.03 | 10.00 | 10.99 | 10.92 | |
| Dose (mL kg−1) | 4.0 | 6.0 | 16.0 | 22.0 | 80.0 | 100.0 | ||
| AL | pH target | 9.00 | 9.50 | 10.00 | 11.00 | ||||
| AS sample ID | DS1 | PS1 | DS1 | PS2 | DS1 | PS1 | DS1 | PS1 | |
| KOH (g kg−1) | 3.0 | 4.0 | 5.0 | 7.0 | 8.0 | 12.0 | 14.0 | 21.0 | |
| pH reached | 9.01 | 9.01 | 9.53 | 9.50 | 10.02 | 10.00 | 11.01 | 11.09 | |
| E. coli (CFU g−1) | 4.50(±0.71) × 102 | 2.90(±1.60) × 105 | <100 | <100 | <100 | <100 | 0.50(±0.71) × 102 | n.a. | |
| AC | pH target | 5.50 | 5.00 | 4.50 | 3.50 | ||||
| AS sample ID | DS1 | PS2 | DS1 | PS2 | DS1 | PS1 | DS1 | PS1 | |
| H2SO4 (mL kg−1) | 6.00 | 6.40 | 6.20 | 8.00 | 6.60 | 9.08 | 7.00 | 14.00 | |
| pH reached | 5.60 | 5.51 | 5.33 | 5.15 | 4.44 | 4.95 | 3.45 | 3.51 | |
| E. coli (CFU g−1) | 1.00(±1.00) × 102 | 2.39(±2.00) × 104 | 1.50(±1.97) × 102 | 1.50(±3.68) × 102 | 1.00(±0.63) × 102 | <100 | <100 | <100 | |
| Slurry Type | Treatment | Escherichia coli (CFU g−1) | ||
|---|---|---|---|---|
| Storage Time | ||||
| t = 0 d | t = 15 d | t = 60 d | ||
| DS (sample DS2) | RS | 3.22(±1.69) × 103 | 1.57(±2.54) × 103 | ˂100 |
| AC | <100 | - | <100 | |
| AL | <100 | <100 | <100 | |
| NE | <100 | <100 | <100 | |
| PS (sample PS3) | RS | 5.96(±7.49) × 105 | 2.33(±1.53) × 102 | <100 |
| AC | <1000 | <100 | <100 | |
| AL | <100 | <100 | <100 | |
| NE | <100 | <100 | <100 | |
| DS | Time (Days) | pH | EC (mS cm−1) | DM (g kg−1) | g kg−1(DM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | TN | NH4+-N | P | K | |||||
| RS | 0 | 7.13 h | 14.13 g | 97.8 k | 421.4 a | 36.1 a | 16.9 a | 7.2 c,d | 27.4 e,f |
| 15 | 7.19 h | 12.83 g,h | 123.5 i | 396.6 b,c | 30.4 d | 11.5 d | 8.1 a,b,c | 26.4 f | |
| 30 | 7.75 f | 11.85 h,i | 161.1 f | 383.0 d | 29.8 d | 8.3 f | 8.3 a,b | 29.9 e | |
| 60 | 8.95 c | 11.38 i | 218.0 c | 371.8 e | 22.4 g | 3.9 i | 8.8 a | 27.9 e,f | |
| AC | 0 | 5.05 l | 18.13 e,f | 108.2 j | 405.4 b | 33.8 b,c | 15.3 b,c | 6.6 d,e | 23.8 g |
| 15 | 5.28 k | 17.53 f | 146.8 g | 396.52 c | 33.9 b | 15.2 c | 6.7 d,e | 22.4 g | |
| 30 | 5.70 j | 20.43 b,c | 191.0 d | 395.13 c | 33.6 b,c | 15.0 c | 7.1 d,e | 23.5 g | |
| 60 | 6.76 i | 21.90 a | 276.5 a | 393.15 c | 32.5 b,c | 15.1 c | 6.1 e | 23.2 g | |
| AL | 0 | 9.23 a | 19.47 c,d | 101.7 j,k | 393.4 c | 32.8 b,c | 15.7 b | 6.1 e | 59.9 b,c |
| 15 | 8.28 d | 18.23 d,e,f | 133.6 h | 372.4 e | 27.2 e | 8.7 f | 6.9 d,e | 59.7 c | |
| 30 | 8.35 d | 19.20 c,d,e | 160.6 f | 371.7 e | 24.3 f | 5.9 h | 7.2 c,d | 67.0 a | |
| 60 | 9.27 a | 20.20 b,c | 215.7 c | 362.5 f | 20.3 h | 3.4 j | 7.3 b,c,d | 62.2 b | |
| NE | 0 | 7.20 h | 21.23 a,b | 107.2 j,k | 372.9 e | 32.3 c | 15.1 c | 6.5 d,e | 60.6 b,c |
| 15 | 7.47 g | 18.80 d,e,f | 137.2 g,h | 355.6 f,g | 27.0 e | 9.8 e | 6.5 d,e | 56.9 d | |
| 30 | 8.04 e | 21.77 a | 178.2 e | 349.2 g | 23.8 f,g | 6.5 g | 7.5 b,c,d | 68.0 a | |
| 60 | 9.08 b | 22.29 a | 257.3 b | 348 g | 18.7 i | 3.3 j | 7.6 b,c,d | 61.8 b,c | |
| Two-way ANOVA | |||||||||
| Slurry treatment (T) | *** | *** | *** | *** | *** | *** | *** | *** | |
| Storage time (t) | *** | *** | *** | *** | *** | *** | *** | *** | |
| T X t | *** | *** | *** | *** | *** | *** | *** | *** | |
| PS | Time (Days) | pH | EC (mS cm−1) | DM (g kg−1) | g kg−1 (DM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | TN | NH4+-N | P | K | |||||
| RS | 0 | 6.69 i | 22.77 e,f | 124.1 i | 465.5 a | 49.6 b,c | 29.9 a,b | 13.0 a,b,c,d,e | 31.6 f |
| 15 | 7.14 f,g | 24.07 e | 141.6 h | 454.7 b | 48.3 c,d,e | 26.9 e | 12.6 b,c,d,e | 36.8 e | |
| 30 | 7.18 f | 21.90 f | 168.9 f,g | 451.2 b | 45.1 f,g | 22.1 g | 14.1 a,b | 39.6 e | |
| 60 | 7.56 e | 21.91 f | 202.3 e | 432.8 e | 42.8 g,h | 16.3 i | 14.6 a | 33.0 f | |
| AC | 0 | 5.14 l | 26.63 d | 126.2 i | 441.6 cd | 49.1 c,d | 29.1 c,d | 11.7 d,e | 31.8 f |
| 15 | 5.46 k | 30.97 c | 164.2 g | 443.0 c | 50.9 a,b,c | 29.5 b,c | 12.3 c,d,e | 30.6 f | |
| 30 | 5.37 k | 31.37 c | 196.8 e | 437.4 de | 52.0 a,b | 30.1 a,b | 12.9 a,b,c,d,e | 30.9 f | |
| 60 | 6.10 j | 33.62 b | 293.2 b | 425.9 f | 52.4 a | 30.6 a | 13.0 a,b,c,d,e | 31.1 f | |
| AL | 0 | 9.41 a | 26.83 d | 125.8 i | 422.1 f,g | 46.2 e,f | 28.3 d | 12.3 c,d,e | 74.3 b,c |
| 15 | 7.98 c | 30.60 c | 162.0 g | 417.6 g,h | 38.4 i | 21.3 g | 11.6 e | 72.9 c,d | |
| 30 | 7.73 d | 26.53 d | 200.8 e | 416.4 h | 33.2 j | 15.7 i | 13.6 a,b,c | 80.0 a | |
| 60 | 8.35 b | 26.49 d | 261.2 c | 394.9 k | 30.9 j | 11.4 j | 14.2 a,b | 75.7 b,c | |
| NE | 0 | 7.09 f,g,h | 30.40 c | 126.7 i | 410.3 i | 46.6 d,e,f | 28.6 d | 12.4 c,d,e | 74.2 b,c |
| 15 | 7.05 g,h | 33.93 a,b | 171.6 f | 409.9 i | 42.2 h | 25.1 f | 12.7 b,c,d,e | 70.2 d | |
| 30 | 6.98 h | 32.20 b,c | 215.2 d | 407.8 i,j | 40.7 i,h | 19.4 h | 13.8 a,b,c | 77.4 a,b | |
| 60 | 8.31 b | 35.89 a | 312.7 a | 403.0 j | 32.4 j | 11.6 j | 13.5 a,b,c,d | 73.9 b,c | |
| Two-way ANOVA | |||||||||
| Slurry treatment (T) | *** | *** | *** | *** | *** | *** | *** | *** | |
| Storage time (t) | *** | *** | *** | *** | *** | *** | *** | *** | |
| T X t | *** | *** | *** | *** | *** | *** | n.s. | *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, J.; Alvarenga, P.; Silva, A.C.; Brito, L.; Tavares, J.; Fangueiro, D. Animal Slurry Sanitization through pH Adjustment: Process Optimization and Impact on Slurry Characteristics. Agronomy 2021, 11, 517. https://doi.org/10.3390/agronomy11030517
Rodrigues J, Alvarenga P, Silva AC, Brito L, Tavares J, Fangueiro D. Animal Slurry Sanitization through pH Adjustment: Process Optimization and Impact on Slurry Characteristics. Agronomy. 2021; 11(3):517. https://doi.org/10.3390/agronomy11030517
Chicago/Turabian StyleRodrigues, Joana, Paula Alvarenga, Ana Carla Silva, Luísa Brito, Jorge Tavares, and David Fangueiro. 2021. "Animal Slurry Sanitization through pH Adjustment: Process Optimization and Impact on Slurry Characteristics" Agronomy 11, no. 3: 517. https://doi.org/10.3390/agronomy11030517
APA StyleRodrigues, J., Alvarenga, P., Silva, A. C., Brito, L., Tavares, J., & Fangueiro, D. (2021). Animal Slurry Sanitization through pH Adjustment: Process Optimization and Impact on Slurry Characteristics. Agronomy, 11(3), 517. https://doi.org/10.3390/agronomy11030517