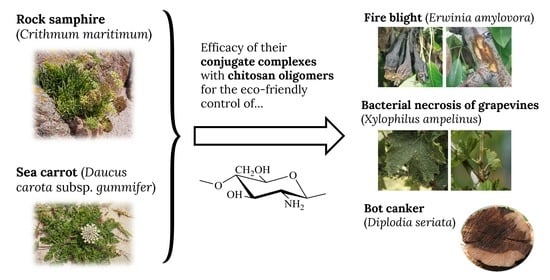
Physicochemical Characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and Their Antimicrobial Activity against Apple Tree and Grapevine Phytopathogens
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Chemicals
2.2. Bacterial and Fungal Isolates
2.3. Preparation of Plant Extracts
2.4. Plant Biomass and Extracts Physicochemical Characterization
2.5. In Vitro Antimicrobial Activity Assessment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Plant Biomass Characterization
3.1.1. Elemental Analysis of Plant Fractions
3.1.2. Thermal Characterization of Flowering Aerial Parts
3.1.3. Vibrational Characterization
3.1.4. On the Usefulness of the Above Physicochemical Techniques
3.2. Extracts Characterization
3.2.1. Phenolic Contents
3.2.2. Active Components by GC-MS Analysis
3.3. In Vitro Antimicrobial Activity
3.3.1. Antibacterial Activity
3.3.2. Antifungal Activity
3.3.3. Comparison with Efficacies Reported in the Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atia, A.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 2011, 5, 3564–3571. [Google Scholar]
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A review of cyanogenic glycosides in edible plants. In Toxicology—New Aspects to This Scientific Conundrum; Larramendy, M.L., Soloneski, S., Eds.; InTechOpen: Rijeka, Croatia, 2016; pp. 179–192. [Google Scholar] [CrossRef] [Green Version]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC: Boca Raton, FL, USA, 2012; p. 3960. [Google Scholar]
- Males, Z.; Zuntar, I.; Nigovic, B.; Plazibat, M.; Vundac, V.B. Quantitative analysis of the polyphenols of the aerial parts of rock samphire -Crithmum maritimum L. Acta Pharm. 2003, 53, 139–144. [Google Scholar] [PubMed]
- Bartnik, M.; Wierzchowska-Renke, K.; Głowniak, P.; Głowniak, K. Phenolic acids in underground parts of Crithmum maritimum L. In Proceedings of the Jubilee XXXth Symposium, Chromatographic Methods of Investigating the Organic Compounds, Katowice, Szczyrk, Poland, 12–14 June 2006; p. 108. [Google Scholar]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of sea fennel (Crithmum maritimum L., Apiaceae) essential oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crop. Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Gil Pinilla, M.; Pérez-Alonso, M.J.; Velasco-Negueruela, A. Volatile constituents from fruits of Daucus carota L., subsp. Gummifer Hooker Fil. J. Essent. Oil Res. 1995, 7, 433–435. [Google Scholar] [CrossRef]
- Valente, J.; Zuzarte, M.; Resende, R.; Goncalves, M.J.; Cavaleiro, C.; Pereira, C.F.; Cruz, M.T.; Salgueiro, L. Daucus carota subsp gummifer essential oil as a natural source of antifungal and anti-inflammatory drugs. Ind. Crop. Prod. 2015, 65, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Houta, O.; Akrout, A.; Najja, H.; Neffati, M.; Amri, H. Chemical composition, antioxidant and antimicrobial activities of essential oil from Crithmum maritimum cultivated in Tunisia. J. Essent. Oil Bear. Plants 2015, 18, 1459–1466. [Google Scholar] [CrossRef]
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burlo, F.; Hernandez, F.; Carbonell-Barrachina, A.A.; Madani, K.; Larbat, R. Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int. J. Food Prop. 2017, 20, 1843–1855. [Google Scholar] [CrossRef] [Green Version]
- Meot-Duros, L.; Le Floch, G.; Magne, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Rossi, P.-G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; de Rocca Serra, D.; Gonny, M.; Bolla, J.-M. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Ozcelik, B.; Kusmenoglu, S.; Turkoz, S.; Abbasoglu, U. Antimicrobial activities of plants from the Apicaceae. Pharm. Biol. 2004, 42, 526–528. [Google Scholar] [CrossRef] [Green Version]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. J. Drug Deliv. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Nawel, M.; El Amine, D.B.; Hocine, A.; Boufeldja, T. Comparative analysis of essential oil components of two Daucus species from Algeria and their antimicrobial activity. Int. Res. J. Biol. Sci. 2013, 2, 22–29. [Google Scholar]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Glamoclija, J.; Sokovic, M.; Grubisic, D.; Vukojevic, J.; Milinekovic, I.; Ristic, M. Antifungal activity of Crithmum maritimum essential oil and its components against mushroom pathogen Mycogone perniciosa. Chem. Nat. Compd. 2009, 45, 96–97. [Google Scholar] [CrossRef]
- Donat, V.; Biosca, E.G.; Peñalver, J.; López, M.M. Exploring diversity among Spanish strains of Erwinia amylovora and possible infection sources. J. Appl. Microbiol. 2007, 103, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Miñana-Galbis, D.; Merino, S.; Tomás, J. Virulence factors of Erwinia amylovora: A review. Int. J. Mol. Sci. 2015, 16, 12836–12854. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-Q.; Tian, Y.-L.; Wang, L.-M.; Geng, G.-M.; Zhao, W.-J.; Hu, B.-S.; Zhao, Y.-F. Fire blight disease, a fast-approaching threat to apple and pear production in China. J. Integr. Agric. 2019, 18, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Willems, A.; Gillis, M.; Kersters, K.; Van Den Broecke, L.; De Ley, J. Transfer of Xanthomonas ampelina Panagopoulos 1969 to a new genus, Xylophilus gen. nov., as Xylophilus ampelinus (Panagopoulos 1969) comb. nov. Int. J. Syst. Bacteriol. 1987, 37, 422–430. [Google Scholar] [CrossRef]
- Szegedi, E.; Civerolo, E.L. Bacterial diseases of grapevine. Int. J. Hortic. Sci. 2011, 17. [Google Scholar] [CrossRef] [Green Version]
- Larignon, P.; Fulchic, R.; Cere, L.; Dubos, B. Observation on black dead arm in French vineyards. Phytopathol. Mediterr. 2001, 40, 336–342. [Google Scholar]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clement, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, N.E. Two apple black rot fungi in the United States. Mycologia 2018, 25, 536–548. [Google Scholar] [CrossRef]
- Brown-Rytlewski, D.E.; McManus, P.S. Virulence of Botryosphaeria dothidea and Botryosphaeria obtusa on apple and management of stem cankers with fungicides. Plant Dis. 2000, 84, 1031–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.A. Botryosphaeria diseases of apple and peach in the Southeastern Unites States. Plant Dis. 1986, 70. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and Streptomyces spp. secondary metabolites against three Botryosphaeriaceae species. Antibiotics 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol. Mediterr. 2007, 46, 18–25. [Google Scholar]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoides L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Garcia Camacho, F.; Grima, E.M.; Martínez Sancho, M.; Sánchez Villasclaras, S. Determinación de ácidos grasos en microalgas marinas. Comparación de diversos métodos de extracción de la fracción lipídica. Grasas Aceites 1990, 41, 13–18. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadley, F.M. The Evidence Required to Show Synergistic Action of Insecticides and a Short Cut in Analysis; U.S. Government Printing Office: Washington, DC, USA, 1945. [Google Scholar]
- Ramiah, M.V. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose, and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Sotiroudis, V.T.; Sotiroudis, T.G.; Kolisis, F.N. The potential of biodiesel production from fatty acid methyl esters of some European/Mediterranean and cosmopolitan halophyte seed oils. J. ASTM Int. 2010, 7. [Google Scholar] [CrossRef]
- Sene, C.F.B.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier-transform Raman and Fourier-transform infrared spectroscopy (an investigation of five higher plant cell walls and their components). Plant Physiol. 1994, 106, 1623–1631. [Google Scholar] [CrossRef] [Green Version]
- Abideen, Z.; Ansari, R.; Khan, M.A. Halophytes: Potential source of ligno-cellulosic biomass for ethanol production. Biomass Bioenergy 2011, 35, 1818–1822. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rodríguez-García, I. Lipids classes, fatty acids and carotenes of the leaves of six edible wild plants. Eur. Food Res. Technol. 1999, 209, 313–316. [Google Scholar] [CrossRef]
- Najjaa, H.; Abdelkarim, B.A.; Doria, E.; Boubakri, A.; Trabelsi, N.; Falleh, H.; Tlili, H.; Neffati, M. Phenolic composition of some Tunisian medicinal plants associated with anti-proliferative effect on human breast cancer MCF-7 cells. Eurobiotech J. 2020, 4, 104–112. [Google Scholar] [CrossRef]
- Ksouri, A.; Dob, T.; Belkebir, A.; Krimat, S.; Chelghoum, C. Chemical composition and antioxidant activity of the essential oil and the methanol extract of Algerian wild carrot Daucus carota L. ssp. carota (L.) Thell. J. Mater. Environ. Sci. 2015, 6, 784–791. [Google Scholar]
- Raju, M.; Varakumar, S.; Lakshminarayana, R.; Krishnakantha, T.; Baskaran, V. Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem. 2007, 101, 1598–1605. [Google Scholar] [CrossRef]
- Ngom, S.; Breant, L.; Antheaume, C.; Herrmann, S.; Leick, A.; Muller, J.; Mekideche, N.; Lobstein, A. Anti-inflammatory compounds from Crithmum maritimum. Planta Med. 2009, 75, 940. [Google Scholar] [CrossRef]
- Ben Mustapha, M.; Zardi-Bergaoui, A.; Chaieb, I.; Flamini, G.; Ascrizzi, R.; Ben Jannet, H. Chemical composition and insecticidal activity of Crithmum maritimum L. essential oil against stored-product beetle Tribolium castaneum. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Guerra, I.; Goncalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crop. Prod. 2020, 149. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Pedro, L.G.; Figueiredo, A.C.; Barroso, J.G. Constituents of the essential oil of sea fennel (Crithmum maritimum L.) growing wild in Turkey. J. Med. Food 2006, 9, 128–130. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Cerantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magne, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Ravasco, J.M.J.M.; Monteiro, C.M.; Trindade, A.F. Cyclopropenes: A new tool for the study of biological systems. Org. Chem. Front. 2017, 4, 1167–1198. [Google Scholar] [CrossRef]
- Majdoub, S.; El Mokni, R.; Muradalievich, A.A.; Piras, A.; Porcedda, S.; Hammami, S. Effect of pressure variation on the efficiency of supercritical fluid extraction of wild carrot (Daucus carota subsp. maritimus) extracts. J. Chromatogr. B 2019, 1125. [Google Scholar] [CrossRef]
- Keser, D.; Guclu, G.; Kelebek, H.; Keskin, M.; Soysal, Y.; Sekerli, Y.E.; Arslan, A.; Selli, S. Characterization of aroma and phenolic composition of carrot (Daucus carota ‘Nantes’) powders obtained from intermittent microwave drying using GC–MS and LC–MS/MS. Food Bioprod. Process. 2020, 119, 350–359. [Google Scholar] [CrossRef]
- Tabet Zatla, A.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Costa, J.; Muselli, A. Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J. Essent. Oil Res. 2017, 29, 391–399. [Google Scholar] [CrossRef]
- Bendiabdellah, A.; Dib, M.E.A.; Djabou, N.; Hassani, F.; Paolini, J.; Tabti, B.; Costa, J.; Muselli, A. Daucus carota ssp. hispanicus Gouan. essential oils: Chemical variability and fungitoxic activity. J. Essent. Oil Res. 2014, 26, 427–440. [Google Scholar] [CrossRef]
- Lichtenstein, E.P.; Liang, T.T.; Schulz, K.R.; Schnoes, H.K.; Carter, G.T. Insecticidal and synergistic components isolated from dill plants. J. Agric. Food Chem. 1974, 22, 658–664. [Google Scholar] [CrossRef]
- Gonçalves, M.J.; Cruz, M.T.; Tavares, A.C.; Cavaleiro, C.; Lopes, M.C.; Canhoto, J.; Salgueiro, L. Composition and biological activity of the essential oil from Thapsia minor, a new source of geranyl acetate. Ind. Crop. Prod. 2012, 35, 166–171. [Google Scholar] [CrossRef]
- Khayyat, S.A.; Sameeh, M.Y. Bioactive epoxides and hydroperoxides derived from naturally monoterpene geranyl acetate. Saudi Pharm. J. 2018, 26, 14–19. [Google Scholar] [CrossRef]
- Braga, P.C.; Alfieri, M.; Culici, M.; Dal Sasso, M. Inhibitory activity of thymol against the formation and viability of Candida albicans hyphae. Mycoses 2007, 50, 502–506. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactivity of polyacetylenes in food plants. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 285–306. [Google Scholar] [CrossRef]
- Lee, D.-S.; Woo, J.-Y.; Ahn, C.-B.; Je, J.-Y. Chitosan–hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jung, W.-K.; Je, J.-Y.; Kim, K.-H.; Kim, H.-W.; Lee, J.; Myeong, J.-I.; Lee, D.-S.; Kang, S.-K.; Eom, S.-H. Synergistic antibacterial effect and antibacterial action mode of chitosan-ferulic acid conjugate against methicillin-resistant Staphylococcus aureus. J. Microbiol. Biotechnol. 2016, 26, 784–789. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, D.; Eom, S.-H.; Kim, S.-H.; Oh, J.; Jung, W.K.; Kim, Y.-M. Synergistic antibacterial effects of chitosan-caffeic acid conjugate against antibiotic-resistant acne-related bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef]
- Pineda, M.R.; Vizcaíno, P.S.; García, P.C.M.; Gil, G.J.H.; Durango, R.D.L. Chemical composition and antifungal activity of Piper auritum Kunth and Piper holtonii C. DC. against phytopathogenic fungi. Chil. J. Agric. Res. 2012, 72, 507–515. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Sturtz, G.; Wedge, D.E.; Schrader, K.K.; Duke, S.O. Phytotoxic and antifungal compounds from two Apiaceae species, Lomatium californicum and Ligusticum hultenii, rich sources of Z-ligustilide and apiol, respectively. J. Chem. Ecol. 2005, 31, 1567–1578. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Vicente Lopez-Llorca, L. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Yuan, F.; Liu, F.; Wang, Y.; Gao, Y. Structure and antimicrobial mechanism of ɛ-polylysine–chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014, 70, 427–434. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Pérez-Lebeña, E.; Martín-Ramos, P. On the applicability of chitosan oligomers-amino acid conjugate complexes as eco-friendly fungicides against grapevine trunk pathogens. Agronomy 2021, 11, 324. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal Activity of Chitosan Oligomers–Amino Acid Conjugate Complexes against Fusarium culmorum in Spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Tomić, A.; Petrović, S.; Pavlović, M.; Tzakou, O.; Couladis, M.; Milenković, M.; Vučićević, D.; Lakušić, B. Composition and antimicrobial activity of the rhizome essential oils of two Athamanta turbith subspecies. J. Essent. Oil Res. 2009, 21, 276–279. [Google Scholar] [CrossRef]
- Snene, A.; Mokni, R.E.; Mahdhi, A.; Joshi, R.K.; Hammami, S. Comparative study of essential oils composition and in vitro antibacterial effects of two subspecies of Daucus carota growing in Tunisia. S. Afr. J. Bot. 2020, 130, 366–370. [Google Scholar] [CrossRef]
- Marongiu, B.; Maxia, A.; Piras, A.; Porcedda, S.; Tuveri, E.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Isolation of Crithmum maritimum L. volatile oil by supercritical carbon dioxide extraction and biological assays. Nat. Prod. Res. 2007, 21, 1145–1150. [Google Scholar] [CrossRef]
- Basım, E.; Basım, H. Note: Evaluation of antibacterial activity of essential oil of Rosa damascena on Erwinia amylovora. Phytoparasitica 2004, 32, 409–412. [Google Scholar] [CrossRef]
- Al-Huqail, A.; Behiry, S.; Salem, M.; Ali, H.; Siddiqui, M.; Salem, A. Antifungal, antibacterial, and antioxidant activities of Acacia Saligna (Labill.) H. L. Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.M.; Salem, M.Z.; Abdel-Megeed, A. In-vitro antibacterial activities of alkaloids extract from leaves of Conocarpus lancifolius Engl. J. Pure Appl. Microbiol. 2013, 7, 1903–1907. [Google Scholar]
- Alkan, D.; Yemenicioğlu, A. Potential application of natural phenolic antimicrobials and edible film technology against bacterial plant pathogens. Food Hydrocoll. 2016, 55, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, I.; Valenzuela, M.; Zamorano, N.; Santander, R.; Baez, C.; Madrid, A. Activity of Adesmia boronioides resinous exudate against phytopathogenic bacteria. Nat. Prod. Res. 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Salem, M.Z.; El Shanhorey, N.; Al-Huqail, A.; Ali, H.M.; Behiry, S.I. Eco-friendly wood-biofungicidal and antibacterial activities of various Coccoloba uvifera L. leaf extracts: HPLC analysis of phenolic and flavonoid compounds. BioResources 2020, 15, 4165–4187. [Google Scholar]
- Vanneste, J.L.; Boyd, R.J. Inhibition of Erwinia amylovora and potential antagonistic bacteria by essential oils and natural compounds. Acta Hortic. 2002, 590, 315–317. [Google Scholar] [CrossRef]
- Karami, O.R.; Khodaverdi, M.; Ali, A.F. Antibacterial effect of effective compounds of Satureja hortensis and Thymus vulgaris essential oils against Erwinia amylovora. J. Agric. Sci. Technol. 2010, 12, 35–45. [Google Scholar]
- Kokoskova, B.; Pavela, R.; Pouvova, D. Effectiveness of plant essential oils against Erwinia amylovora, Pseudomonas syringae pv. syringae and associated saprophytic bacteria on/in host plants. J. Plant Pathol. 2011, 93, 133–139. [Google Scholar]
- Cobos, R.; Mateos, R.M.; Alvarez-Perez, J.M.; Olego, M.A.; Sevillano, S.; Gonzalez-Garcia, S.; Garzon-Jimeno, E.; Coque, J.J. Effectiveness of natural antifungal compounds in controlling infection by grapevine trunk disease pathogens through pruning wounds. Appl. Environ. Microbiol. 2015, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Peak | Rt (min) | Area (%) | Tentative Assignments |
|---|---|---|---|
| 11 | 9.842 | 2.78 | benzene, 2-methoxy-4-methyl-1-(1-methylethyl)- (also named methylthymol); 3-methoxy-p-cymene (also named 2-isopropyl-5-methylanisole or tymol methyl ether) |
| 15 | 11.005 | 0.88 | 2-methoxy-4-vinylphenol (or 4-vinylguaiacol); 1-(2-hydroxy-5-methylphenyl)ethanone; 3-methoxyacetophenone |
| 21 | 14.068 | 0.80 | 1,2,3-trimethoxy-5-allylbenzene (or elemicin) |
| 22 | 15.163 | 54.58 | 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene (or apiole) |
| 33 | 18.143 | 0.92 | ethyl 2-(3-hydroxyphenyl)acetate methanol, cyclohexylphenyl-1-(4-hydroxyphenyl)-2-(3-hydroxyphenyl)ethane |
| 49 | 19.170 | 3.78 | falcarinol; propenoic acid, 3-(cycloheptatrien-7-yl-, methyl ester N,N-dimethyl-1H-inden-2-amine |
| 50 | 20.499 | 2.79 | 1-methyl-4-nitrosobenzene; bicyclo[4.2.0]octa-1,3,5-trien-7-ol |
| 51 | 20.777 | 23.83 | 1,2-dimethyl-3-phenylcyclopropene; α-methyl-2-naphthalenemethanol dimethyl; 1,2-diethenyl tricyclo[3.1.0.0(2,4)]hexane-3,6-dicarboxylate |
| Peak | Rt (min) | Area (%) | Tentative Assignments |
|---|---|---|---|
| 6 | 6.219 | 1.12 | 1,6-anhydro-2,4-dideoxy-β-D-ribo-hexopyranose; propanoic acid, 2,2-dimethyl-, hexyl ester; 2-methylbutanal |
| 20 | 11.925 | 22.73–39.68 | (Z)-3,7-dimethyl-2,6-octadien-1-ol, acetate (or geranyl acetate) |
| 22 | 12.519 | 2.70 | caryophyllene; bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-,[1R-(1R*,4Z,9S*)] |
| 26 | 13.254 | 1.87 | 2,6-dimethyl-3,5,7-octatriene-2-ol; geranyl acetate, 2,3-epoxy- |
| 28 | 13.756 | 1.49 | (E,Z)-α-farnesene; 6-epi-shyobunol; epiglobulol |
| 34 | 14.569 | 1.30 | caryophyllene oxide; cyclohexaneethanol, 2-methylene- |
| 40 | 15.528 | 1.35 | 1,2,3,5-cyclohexanetetrol, (1α,2β,3α,5β)-; 4-methyl-5-propyl-nonane; trichloroacetic acid, 4-methylpentyl ester |
| 55 | 19.418 | 2.61 | 4-hydroxy-4-(4,6-dimethylcyclohex-3-enyl)butan-2-one; 3-buten-2-one, 4-(3-hydroxy-6,6-dimethyl-2-methylenecyclohexyl)-; 7,8-epoxy-α-ionone |
| 59 | 19.920 | 0.65 | spiro[4.5]decan-7-one, 1,8-dimethyl-8,9-epoxy-4-isopropyl-; biciclo[4,1,0]heptan-3-ol,3,7,7-trimethyl-, [1S-1α,3α,6α]- |
| 62 | 20.163 | 1.23 | 3-carene; tricyclo[2.2.1.0(2,6)]heptane, 1,3,3-trimethyl- |
| 63 | 20.431 | 1.16 | 5-ethyl-2,4-dimethyl-2-heptene; hexan-3-yl (E)-2-methylbut-2-enoate |
| 84 | 23.201 | 1.33 | hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 86 | 23.566 | 2.42 | anhydro-4,6-dimethyl-3-[p-chlorophenyl]-7-hydroxy-1,2,4-triazolo[1,5-a]pyrimidinium-5-one |
| 91 | 24.593 | 2.38 | (9Z,12Z)-1,3-Dihydroxypropan-2-yl octadeca-9,12-dienoate (or β-monolinolein) |
| 97 | 25.299 | 5.50 | 1,2-dicyclohexyl-1,1-propanedicarbonitrile; 1,6-dibromohexane; 3-methylbut-2-enoic acid, 3,5-dimethylphenyl ester |
| 99 | 25.480 | 4.92 | 3-ethyl-2-butenoic acid, phenyl ester; bromocyclohexane |
| 103 | 25.947 | 1.71 | 3-methyl-but-2-enoic acid, 1,7,7-trimethyl-bicyclo[2.2.1]hept-2-yl ester |
| 107 | 30.192 | 2.52–6.95 | γ-sitosterol |
| Pathogen | Compound | 62.5 | 93.7 | 125 | 187.5 | 250 | 375 | 500 | 750 | 1000 | 1500 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. amylovora | COS | + | + | + | + | + | + | + | + | + | - |
| C. maritimum | + | + | + | + | + | + | + | + | + | - | |
| D. carota | + | + | + | + | + | + | + | + | + | - | |
| Apiole | + | + | + | + | + | + | + | + | + | - | |
| Geranyl acetate | + | + | + | + | + | + | + | + | + | + | |
| COS-apiole | + | + | + | + | + | + | + | - | - | - | |
| COS-geranyl acetate | + | + | + | + | + | + | + | + | - | - | |
| COS-C. maritimum | + | + | + | + | - | - | - | - | - | - | |
| COS-D. carota | + | + | + | + | + | + | - | - | - | - | |
| X. ampelinus | COS | + | + | + | + | + | + | + | + | + | - |
| C. maritimum | + | + | + | + | + | + | + | - | - | - | |
| D. carota | + | + | + | + | + | + | + | + | - | - | |
| Apiole | + | + | + | + | + | + | + | + | + | - | |
| Geranyl acetate | + | + | + | + | + | + | + | + | + | - | |
| COS-apiole | + | + | + | + | + | + | + | - | - | - | |
| COS-geranyl acetate | + | + | + | - | - | - | - | - | - | - | |
| COS-C. maritimum | + | + | + | + | + | - | - | - | - | - | |
| COS-D. carota | + | + | + | + | + | + | - | - | - | - |
| Effective Concentration | COS | Apiole | Geranyl Acetate | D. carota | C. maritimum | COS-Apiol | COS-Geranyl Acetate | COS-D. carota | COS-C. maritimum |
|---|---|---|---|---|---|---|---|---|---|
| EC50 | 744 | 807 | 147 | − | 832 | 333 | 68 | 269 | 75 |
| EC90 | 1180 | 1353 | 272 | − | 2933 | 822 | 113 | 633 | 331 |
| SF | 1.53 | 3.91 | − | 5.08 |
| Phytochemical | Product Type | Microorganisms | Effectiveness | Ref |
|---|---|---|---|---|
| Apiole | EO from rhizomes of Athamanta turbith 33–49% apiole | Bacteria: | MIC (mg·mL−1) | [67] |
| E. coli ATCC 25922 | 43.3 | |||
| P. aeruginosa ATCC 27853 | >86.6 | |||
| S. aureus ATCC 25923 | 43.3 | |||
| S. epidermidis ATCC 12228 | 86.6 | |||
| M. luteus ATCC 10240 | 43.3 | |||
| K. pneumoniae NCIMB 9111 | >86.6 | |||
| Fungi: | ||||
| C. albicans ATCC 10259 | >86.6 | |||
| EO from aerial parts of Piper holtonii 57% apiole | Fungi: | IC50 (μg·mL−1) | [61] | |
| Colletotrichum acutatum | <50 | |||
| Botryodiplodia theobromae | 36.16 | |||
| Geranyl acetate | EO of lemongrass varieties 0.5–1% geranyl ac. | Bacteria: | MIC (μg·mL−1) | [55] |
| P. aeruginosa | 4.5–9 | |||
| S. aureus | 4.5–18 | |||
| EO from aerial parts of Thapsia minor: 83% geranyl acetate | Fungi: | MIC (μL·mL−1) | [54] | |
| C. albicans ATCC 10231 | >20 | |||
| C. tropicalis ATCC 13803 | >20 | |||
| C. krusei H9 | 10–20 | |||
| C. guillermondii MAT23 | 1.25 | |||
| C. parapsilosis ATCC 90018 | 2.5–5 | |||
| T. rubrum CECT 2794 | 0.32 | |||
| M. gypseum CECT 2905 | 0.64 | |||
| M. canis FF1 | 0.32–0.64 | |||
| C. neoformans CECT1078 | 0.32 | |||
| E. floccosum FF9 | 0.16 | |||
| A. flavus F44 | >20 | |||
| A. niger ATCC16404 | >20 | |||
| A. fumigatus ATCC 46645 | 10–20 | |||
| D. carota subsp. gummifer | EO of aerial parts, 37% geranyl acetate | Fungi: | MIC (μL·mL−1) | [8] |
| C. albicans ATCC 10231 | >20 | |||
| C. tropicalis ATCC 13803 | 10 | |||
| C. krusei H9 | >20 | |||
| C. guillermondii MAT 23 | 1.25 | |||
| C. parapsilosis ATCC 90018 | >20 | |||
| T. rubrum CECT 2794 | 0.32 | |||
| M. gypseum CECT 2908 | 0.64 | |||
| M. canis FF1 | 0.64 | |||
| E. floccosum FF9 | 0.32 | |||
| A. flavus F44 | >20 | |||
| A. niger ATCC 16404 | 10 | |||
| A. fumigatus ATCC 46645 | 2.5 | |||
| EO of aerial parts 52–77% geranyl ac. | Bacteria: | MIC (mg·mL−1) | [15] | |
| E. coli ATCC 25922 | >6.0 | |||
| P. aeruginosa ATCC 27853 | >6.0 | |||
| S. aureus ATCC 25923 | 5.1 | |||
| B. cereus ATCC 9634 | 3.8 | |||
| E. faecalis ATCC 29212 | 4.3 | |||
| K. pneumoniae ATCC 10031 | >6.0 | |||
| D. carota subsp. hispidus | EO of aerial parts | Bacteria: | MIC (mg·mL−1) | [68] |
| E. coli ATCC 35218 | 1.25 | |||
| S. aureus ATCC 25923 | 2.5 | |||
| E. faecalis ATCC 29212 | 1.25 | |||
| C. maritimum | Plant extract and EO of aerial parts | Bacteria: | IC50 = 0.47 mg·mL−1 (Kélibia) and 3.3 mg·mL−1 (Monastir) | [30] |
| E. coli ATCC 10536 | ||||
| P. aeruginosa ATCC 9027 | ||||
| S. aureus ATCC 6538 | ||||
| B. cereus ATCC 11778 | ||||
| Hydromethanolic extract of aerial parts | Fungi: | MIC (μg·mL−1) | [10] | |
| E. coli ATCC 25922 | 0.11 | |||
| C. albicans ATCC 10231 | 0.11 | |||
| Hexane extract of leaves | Bacteria: | MIC (μg·mL−1) | [47] | |
| E. coli BCC 3.08.001 and ATCC 4157 | - | |||
| B. cereus BCC 3.05.002 | 50 | |||
| M. luteus ATCC 10240 | 50 | |||
| E. carotovora BCC 3.08.031 | - | |||
| Fungi: | ||||
| C. albicans BCC 3.08.036. | - | |||
| Volatile oils of leaves | Fungi: | MIC (μg·mL−1) | [69] | |
| C. albicans ATCC 10231 | 2.5–5 | |||
| C. guillermondii MAT23 | 0.32–2.5 | |||
| C. neoformans CECT 1078 | 0.32–0.64 | |||
| E. floccosum FF9 | 0.08–0.32 | |||
| T. rubrum CECT 2794 | 0.08–0.32 | |||
| M. gypseum CECT 2908 | 0.08–1.25 | |||
| M. canis FF1 | 0.08–0.64 | |||
| Essential oil of roots | M. perniciosa | MIQ = 1 μL/disc | [17] |
| Phytochemical | Effective Dose | Ref. |
|---|---|---|
| EO of Rosa damascena flowers | MCB = 1386.5 μg·mL−1 | [70] |
| Water extract (7.4% w/w) of Acacia saligna flowers | MIC = 300 μg·mL−1 | [71] |
| Alkaloids extract from Conocarpus lancifolius leaves | MIC > 200 μg·mL−1 | [72] |
| Phenolic extracts from: | MIC (mg·mL−1) | [73] |
| Syzygium aromaticum | 10.2 | |
| Origanum vulgare | 91% inhibition at 41.0 | |
| Cynara cardunculus var. scolymus stem | 48% inhibition at 41.0 | |
| Juglans regia shells | No inhibition | |
| Exudate from Adesmia boronioides (8.5% resin/fresh plant) | MIC = 64 μg·mL−1 | [74] |
| Alkaloids extr. from Peganum harmala seeds | MIC = 50 μg·mL−1 | [75] |
| Extracts from Coccoloba uvifera leaves: | Diam. inhib. zone (mm) at 2500 μg·mL−1 | [76] |
| Aqueous | 8 ± 1 | |
| Acetone | 10 ± 1 | |
| Ethanol | 14 | |
| EO from: | Diam. inhib. zone (mm), concentr. N/A | [77] |
| Cinnamomum zeylanicum | 31.2 | |
| Laurus nobilis | 22 | |
| Thymus vulgaris | 20.6 | |
| Syzygium aromaticum | 18 | |
| Pinus spp. | 17 | |
| Cymbogon citratus | 13 | |
| Mentha spicata | 13 | |
| Melaleuca alternifolia | 12 | |
| EO from aerial parts of flowering: | Diam. inhib. zone (mm), concentr. N/A | [78] |
| Thymus vulgaris | 25 | |
| Satureja hortensis | 25 | |
| EOs extr. by steam or hydrodistillation from: | Diam. inhib. zone (cm), concentr. N/A | [79] |
| Melissa officinalis flowers/leaves | 6.17–8.7 | |
| Mentha arvensis aerial part | 7.67–12.7 | |
| Nepeta cataria flowering tops | 12.1–24.00 | |
| Origanum compactum aerial part | 21.33–29.3 | |
| Origanum vulgare aerial part | 14.50–25.5 | |
| Thymus vulgaris aerial part | 14.33–37.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; Buzón-Durán, L.; Andrés-Juan, C.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical Characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and Their Antimicrobial Activity against Apple Tree and Grapevine Phytopathogens. Agronomy 2021, 11, 886. https://doi.org/10.3390/agronomy11050886
Sánchez-Hernández E, Buzón-Durán L, Andrés-Juan C, Lorenzo-Vidal B, Martín-Gil J, Martín-Ramos P. Physicochemical Characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and Their Antimicrobial Activity against Apple Tree and Grapevine Phytopathogens. Agronomy. 2021; 11(5):886. https://doi.org/10.3390/agronomy11050886
Chicago/Turabian StyleSánchez-Hernández, Eva, Laura Buzón-Durán, Celia Andrés-Juan, Belén Lorenzo-Vidal, Jesús Martín-Gil, and Pablo Martín-Ramos. 2021. "Physicochemical Characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and Their Antimicrobial Activity against Apple Tree and Grapevine Phytopathogens" Agronomy 11, no. 5: 886. https://doi.org/10.3390/agronomy11050886
APA StyleSánchez-Hernández, E., Buzón-Durán, L., Andrés-Juan, C., Lorenzo-Vidal, B., Martín-Gil, J., & Martín-Ramos, P. (2021). Physicochemical Characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and Their Antimicrobial Activity against Apple Tree and Grapevine Phytopathogens. Agronomy, 11(5), 886. https://doi.org/10.3390/agronomy11050886