An Environmentally Friendly Soil Amendment for Enhancing Soil Water Availability in Drought-Prone Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.1.1. Properties of ATP
2.1.2. Soil Samples
2.2. Laboratory Measurements
2.2.1. Soil Water Retention Curve
2.2.2. Soil Physical Properties
2.2.3. Soil Pore Size Distribution
2.2.4. Soil Saturated Hydraulic Conductivity
2.2.5. Soil Aggregate Measurement
2.3. Statistical Analysis
3. Results
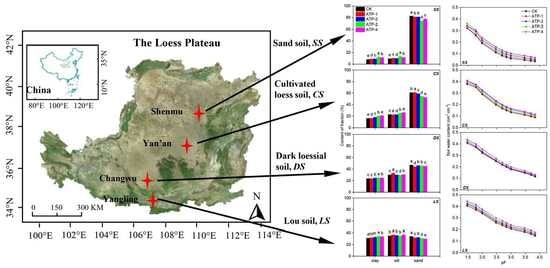
3.1. Soil Physical Properties
3.2. Soil Water Retention Curve and Water Characteristic Parameters
3.3. Soil Pore Size Distribution
3.4. Soil Saturated Hydraulic Conductivity
3.5. Soil Aggregate Stability
4. Discussion
4.1. The Effect of ATP on Soil Hydraulic Properties
4.2. The Effect of ATP on Soil Pore Characteristics and Aggregate Structure and Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, R.; Scholes, R.; Ash, N. Ecosystems and Human Well-being: Current State and Trends: Findings of the Condition and Trends Working Group. In The Millennium Ecosystem Assessment Series; Island Press: Washington, DC, USA, 2005; p. 917. [Google Scholar]
- Minckley, T.A.; Turner, D.S.; Weinstein, S.R. The relevance of wetland conservation in arid regions: A re-examination of vanishing communities in the American Southwest. J. Arid. Environ. 2013, 88, 213–221. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, C.X.; Hasi, E.; Dong, Z.B. Has the Three Norths Forest Shelterbelt Program solved the desertification and dust storm problems in arid and semiarid China? J. Arid. Environ. 2010, 74, 13–22. [Google Scholar] [CrossRef]
- Moska, P.; Jary, Z.; Adamiec, G.; Bluszcz, A. High resolution dating of loess profile from Strzyżów (Horodło Plateau-Ridge, Volhynia Upland). Quatern. Int. 2018, 502, 18–29. [Google Scholar] [CrossRef]
- Wang, Y.; Magliulo, V.; Yan, W.; Shangguan, Z. Assessing land surface drying and wetting trends with a normalized soil water index on the Loess Plateau in 2001–2016. Sci. Total Environ. 2019, 676, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shao, M. Advances and perspectives on soil water research in China’s Loess Plateau. Earth-Sci. Rev. 2019, 199, 102962. [Google Scholar] [CrossRef]
- Cai, X.; McKinney, D.C.; Rosegrant, M.W. Sustainability analysis for irrigation water management in the Aral Sea region. Agric. Syst. 2003, 76, 1043–1066. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Yang, J.; Yan, L.; Shi, Y. Effects of super absorbent resin on soil characteristics in dry-land wheat. Adv. J. Food Sci. Technol. 2014, 6, 480–483. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, Y.; Feng, H.; Peng, X.; Si, B. Using the double-exponential water retention equation to determine how soil pore-size distribution is linked to soil texture. Soil Tillage Res. 2016, 156, 119–130. [Google Scholar] [CrossRef]
- Ge, N.; Wei, X.; Wang, X.; Liu, X.; Shao, M.; Jia, X.; Zhang, Q. Soil texture determines the distribution of aggregate-associated carbon, nitrogen and phosphorous under two contrasting land use types in the Loess Plateau. Catena 2019, 172, 148–157. [Google Scholar] [CrossRef]
- Pan, F.F.; Peters-Lidard, C.D.; King, A.W. Inverse method for estimating the spatial variability of soil particle size distribution from observed soil moisture. J. Hydrol. Eng. 2010, 15, 931–938. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, M.; Jia, X.; Zhang, C. Particle size distribution of soils (0–500cm) in the Loess Plateau, China. Geoderma Reg. 2016, 7, 251–258. [Google Scholar] [CrossRef]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lin, Y.; Shi, W.; Song, Y.; He, X. Balancing green and grain trade. Nat. Geosci. 2015, 8, 739–741. [Google Scholar] [CrossRef]
- Feng, X.; Fu, B.; Piao, S.; Wang, S.; Ciais, P.; Zeng, Z.; Wu, B. Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Change 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, D.; Yang, Y.; Piao, S.; Yang, H.; Lei, H.; Fu, B. Excessive afforestation and soil drying on china’s loess plateau. J. Geophys. Res-Biogeo. 2018, 123, 923–935. [Google Scholar] [CrossRef]
- Liang, H.; Xue, Y.; Li, Z.; Wang, S.; Wu, X.; Gao, G.; Fu, B. Soil moisture decline following the plantation of Robinia pseudoacacia forests: Evidence from the Loess Plateau. For. Ecol. Manag. 2018, 412, 62–69. [Google Scholar] [CrossRef]
- Nadler, A.; Perfect, E.; Kay, B.D. Effect of polyacrylamide application on the stability of dry and wet aggregates. Soil Sci. Soc. Am. J. 1996, 60, 555–561. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.J.; Spokas, K.A.; Feyereisen, G. Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 2016, 142, 136–144. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Seggiani, M.; Cinelli, P.; Elnaby, H.; Azaam, M.M. Swelling capacity of sugarcane bagasse-g-poly (acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer. Eur. Polym. J. 2020, 133, 109769. [Google Scholar] [CrossRef]
- Garland, T.O.; Patterson, M.W.H. Six cases of acrylamide poisoning. Br. Med. J. 1967, 4, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar]
- Wang, J.; Xia, K.; Waigi, M.G.; Gao, Y.; Odinga, E.S.; Ling, W.; Liu, J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons. Environ. Int. 2018, 121, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Wang, R. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2019, 229, 115512. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Siebecker, M.G.; Gou, W.; Li, W. EXAFS investigation of Ni (II) sorption at the palygorskite-solution interface: New insights into surface-induced precipitation phenomena. Geochim. Cosmochim. Acta 2021, 314, 85–107. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Gan, F.; Zhou, J.; Wang, H.; Du, C.; Chen, X. Removal of phosphate from aqueous solution by thermally treated natural palygorskite. Water Res. 2009, 43, 2907–2915. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Jin, Q.; Wang, X.; Yang, J. Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J. Hazard. Mater. 2007, 143, 541–548. [Google Scholar] [CrossRef]
- Yin, H.; Yan, X.; Gu, X. Evaluation of thermally-modified calcium-rich attapulgite as a low-cost substrate for rapid phosphorus removal in constructed wetlands. Water Res. 2017, 115, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dai, J.; Wang, L.; Li, Y.; Song, Y. First principles study of structural stability against the distribution of Mg and Al atoms and adsorption behaviors of heavy metals of attapulgite. Comput. Mater. Sci. 2019, 169, 109106. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, W.; Lin, H. Effect of palygorskite adding NPK fertilizer on plant dry matter accumulation and polysaccharide content of radix hedysari. Med. Plants 2010, 4, 5–8. [Google Scholar]
- Qin, S.; Wu, Z.; Rasool, A.; Li, C. Synthesis and characterization of slow-release nitrogen fertilizer with water absorbency: Based on poly (acrylic acid-acrylic amide)/Na-bentonite. J. Appl. Polym. Sci. 2012, 126, 1687–1697. [Google Scholar] [CrossRef]
- Guan, Y.; Song, C.; Gan, Y.; Li, F. Increased maize yield using slow-release attapulgite-coated fertilizers. Agron. Sustain. Dev. 2014, 34, 657–665. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Joseph, S.D.; Li, F.; Bai, Y.; Shang, Z.; Rawal, A.; Long, R.J. Pyrolysis of attapulgite clay blended with yak dung enhances pasture growth and soil health: Characterization and initial field trials. Sci. Total Environ. 2017, 607, 184–194. [Google Scholar] [CrossRef]
- Zhu, W.L.; Cui, L.H.; OuYang, Y.; Long, C.F.; Tang, X.D. Kinetic adsorption of ammonium nitrogen by substrate materials for constructed wetlands. Pedosphere 2011, 21, 454–463. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. Adsorption of dyes onto palygorskite and its composites: A review. J. Environ. Chem. Eng. 2016, 4, 1274–1294. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Su, J.; Wu, J.; Xia, Y.; Zhu, L.; Zhang, D. An efficient and no pollutants deproteinization method for polysaccharide from Arca granosa by palygorskite adsorption treatment. J. Clean. Prod. 2019, 226, 781–792. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, L.; Bao, J.; Firmin, K.A.; Zong, W. Attapulgite enhances methane production from anaerobic digestion of pig slurry by changing enzyme activities and microbial community. Renew. Energy 2020, 145, 222–232. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S. A new approach to modification of natural adsorbent for heavy metal adsorption. Bioresour. Technol. 2008, 99, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Thomas, K.M. Adsorption of aqueous metal ions on oxygen and nitrogen functionalized nanoporous activated carbons. Langmuir 2005, 21, 3892–3902. [Google Scholar] [CrossRef]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of ph-responsive lignin-based nanocapsules for controlled release of hydrophobic molecules. ACS Sustain. Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Xing, B. Sorption of naphthalene and phenanthrene by soil humic acids. Environ. Pollut. 2001, 111, 303–309. [Google Scholar] [CrossRef]
- Falayi, T.; Ntuli, F. Removal of heavy metals and neutralisation of acid mine drainage with un-activated attapulgite. J. Ind. Eng. Chem. 2014, 20, 1285–1292. [Google Scholar] [CrossRef]
- Sheffield, J.; Wood, E.F. Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim. Dyn. 2007, 31, 79–105. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Zangiabadi, M.; Gorji, M.; Shorafa, M.; Khorasani, S.K.; Saadat, S. Effects of soil pore size distribution on plant available water and least limiting water range as soil physical quality indicators. Pedosphere 2020, 30, 253–262. [Google Scholar] [CrossRef]
- Tian, X.; Fan, H.; Wang, J.; Ippolito, J.; Li, Y.; Feng, S.; Wang, K. Effect of polymer materials on soil structure and organic carbon under drip irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Liebig, M.A.; Tanaka, D.L.; Wienhold, B.J. Tillage and cropping effects on soil quality indicators in the northern Great Plains. Soil Tillage Res. 2004, 78, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Haverkamp, R.; Leij, F.J.; Fuentes, C. Soil water retention. Soil Sci. Soc. Am. J. 2005, 69, 95–104. [Google Scholar] [CrossRef]
- Amoakwah, E.; Frimpong, K.A.; Okaeanti, D. Soil water retention, air flow and pore structure characteristics after corn cob biochar application to a tropical sandy loam. Geoderma 2017, 307, 189–197. [Google Scholar] [CrossRef]
- Gardner, W.R. Availability and measurement of soil water. Water Deficits Plant Growth 1968, 1, 107–135. [Google Scholar]
- Aghaalikhani, M.; Gholamhoseini, M.; Dolatabadian, A.; Khodaei-Joghan, A.; Asilan, K.S. Zeolite influences on nitrate leaching, nitrogen-use efficiency, yield and yield components of canola in sandy soil. Arch. Agron. Soil Sci. 2012, 58, 1149–1169. [Google Scholar] [CrossRef]
- Liu, H.; Lei, T.W.; Zhao, J.; Yuan, C.P.; Fan, Y.T.; Qu, L.Q. Effects of rainfall intensity and antecedent soil water content on soil infiltrability under rainfall conditions using the run off-on-out method. J. Hydrol. 2011, 396, 24–32. [Google Scholar] [CrossRef]
- Biswas, A.; Chau, H.W.; Bedard-Haughn, A.K.; Si, B.C. Factors controlling soil water storage in the hummocky landscape of the Prairie Pothole Region of North America. Can. J. Soil Sci. 2012, 92, 649–663. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, H.; Li, T.; Hou, R.; Liu, D.; Ji, Y.; Yang, L. Effects of biochar addition on soil hydraulic properties before and after freezing-thawing. Catena 2019, 176, 112–124. [Google Scholar] [CrossRef]
- Chamberlain, E.J.; Gow, A.J. Effect of freezing and thawing on the permeability and structure of soils. Eng. Geol. 1979, 13, 73–92. [Google Scholar] [CrossRef]
- Hillel, D.; Tadmor, N. Water regime and vegetation in the Central Negev Highlands of Israel. Ecology 1962, 43, 33–41. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, F.; Hu, K.; Kidron, G.J. Biocrusts reduce surface soil infiltrability and impede soil water infiltration under tension and ponding conditions in dryland ecosystem. J. Hydrol. 2018, 568, 792–802. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, X.; Gao, X.; Zhang, S.; Wu, P. The effects of long-term fertilizer applications on soil organic carbon and hydraulic properties of a loess soil in China. Land Degrad. Dev. 2016, 27, 60–67. [Google Scholar] [CrossRef]
- Gao, L.; Wang, B.; Li, S.; Wu, H.; Wu, X.; Liang, G.; Gong, D.; Zhang, X.; Cai, D.; Degré, A. Soil wet aggregate distribution and pore size distribution under different tillage systems after 16 years in the Loess Plateau of China. Catena 2019, 173, 38–47. [Google Scholar] [CrossRef]
- Xu, X.; Nieber, J.L.; Gupta, S.C. Compaction effects on the gas diffusion coefficients in soils. Soil Sci. Soc. Am. J. 1992, 56, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- Mamedov, A.; Shainberg, I.; Levy, G. Wetting rate and sodicity effects on interrill erosion from semi-arid Israeli soils. Soil Tillage Res. 2002, 68, 121–132. [Google Scholar] [CrossRef]
- Wakindiki, I.I.C.; Ben-Hur, M. Soil mineralogy and texture effects on crust micromorphology, infiltration, and erosion. Soil Sci. Soc. Am. J. 2002, 66, 897–905. [Google Scholar] [CrossRef]
- Ruiz-Vera, V.M.; Wu, L. Influence of sodicity, clay mineralogy, prewetting rate, and their interaction on aggregate stability. Soil Sci. Soc. Am. J. 2006, 70, 1825–1833. [Google Scholar] [CrossRef]
- Reichert, J.M.; Norton, L.D.; Favaretto, N.; Huang, C.; Blume, E. Settling velocity, aggregate stability, and interrill erodibility of soils varying in clay mineralogy. Soil Sci. Soc. Am. J. 2009, 73, 1369–1377. [Google Scholar] [CrossRef] [Green Version]
- Kemper, W.D.; Rosenau, R.C.; Dexter, A.R. Cohesion development in disrupted soils as affected by clay and organic matter content and temperature. Soil Sci. Soc. Am. J. 1987, 51, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shi, W.; Shao, H.; Shao, M. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef]
- Lentz, R.D. Polyacrylamide and biopolymer effects on flocculation, aggregate stability, and water seepage in a silt loam. Geoderma 2015, 241, 289–294. [Google Scholar] [CrossRef]





| Soil Types | BD (g·cm−3) | IWC (%) | SOM (%) | Particle Composition (%) | Soil Texture | ||
|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | |||||
| Lou soil (LS) | 1.33 | 3.23 | 15.4 ± 0.4 | 31.0 | 35.0 | 34.0 | Clay loam |
| Dark loessial soil (DS) | 1.38 | 3.69 | 10.9 ± 0.3 | 23.4 | 29.8 | 46.8 | Loam |
| Cultivated loess soil (CS) | 1.38 | 3.58 | 7.9 ± 0.4 | 16.3 | 23.0 | 60.7 | Sandy loam |
| Sandy soil (SS) | 1.57 | 2.27 | 6.8 ± 0.2 | 8.1 | 9.4 | 82.5 | Loamy sand |
| Soil Types | Treatments | Clay (%) | Silt (%) | Sand (%) | TP (%) | CP (%) |
|---|---|---|---|---|---|---|
| LS | CK | 31.0 ± 0.1 Da | 35.0 ± 0.0 Ba | 34.0 ± 0.1 Ad | 50.8 ± 0.7 Cd | 46.9 ± 1.8 Aa |
| ATP-1 | 31.8 ± 0.1 CDa | 36.6 ± 0.1 Aa | 31.6 ± 0.2 Cd | 51.2 ± 3.1 Bc | 47.2 ± 1.8 Aa | |
| ATP-2 | 32.5 ± 1.0 Ca | 35.4 ± 0.9 Ba | 32.2 ± 0.1 Bd | 51.3 ± 1.3 ABd | 48.3 ± 1.7 Aa | |
| ATP-3 | 34.5 ± 0.0 Aa | 34.6 ± 0.1 Ba | 30.8 ± 0.0 Dd | 51.3 ± 1.1 ABd | 48.7 ± 1.5 Aa | |
| ATP-4 | 34.7 ± 0.2 Ba | 36.7 ± 0.2 Aa | 28.6 ± 0.4 Ed | 52.0 ± 2.0 Ac | 48.4 ± 2.2 Ab | |
| DS | CK | 23.4 ± 0.2 Cb | 29.8 ± 0.3 Cb | 46.8 ± 0.4 Ac | 52.0 ± 0.9 Ac | 47.0 ± 1.2 Aa |
| ATP-1 | 23.3 ± 0.1 Cb | 32.8 ± 0.1 Ab | 43.9 ± 0.1 Dc | 52.7 ± 0.4 Bb | 46.9 ± 0.7 Aa | |
| ATP-2 | 24.4 ± 0.0 Bb | 29.7 ± 0.0 Cb | 45.9 ± 0.0 Bc | 52.6 ± 0.8 bCc | 47.3 ± 1.3 Aa | |
| ATP-3 | 24.9 ± 0.0 Ab | 29.5 ± 0.0 Db | 45.6 ± 0.0 Bc | 53.0 ± 1.2 CDc | 48.1 ± 1.1 Aa | |
| ATP-4 | 25.5 ± 0.0 Bb | 30.5 ± 0.0 Bb | 43.9 ± 0.0 Cc | 52.8 ± 0.3 Db | 47.8 ± 0.3 Abc | |
| CS | CK | 16.3 ± 0.1 Ec | 23.0 ± 0.0 Cc | 60.7 ± 0.1 Bb | 50.6 ± 0.7 Ab | 47.9 ± 0.9 Ca |
| ATP-1 | 16.4 ± 0.0 Dc | 22.6 ± 0.0 Ec | 60.9 ± 0.1 Ab | 50.9 ± 0.5 Bb | 48.5 ± 0.9 BCa | |
| ATP-2 | 18.1 ± 0.0 Cc | 22.9 ± 0.0 Dc | 59.1 ± 0.0 Cb | 51.0 ± 0.9 Cb | 49.1 ± 1.0 ABCa | |
| ATP-3 | 20.5 ± 0.0 Bc | 25.1 ± 0.0 Bc | 54.5 ± 0.0 Db | 51.3 ± 0.3 Cb | 49.7 ± 1.1 ABa | |
| ATP-4 | 21.1 ± 0.0 Ac | 26.4 ± 0.0 Ac | 52.5 ± 0.0 Eb | 50.9 ± 2.5 Da | 50.8 ± 0.3 Aa | |
| SS | CK | 8.1 ± 0.0 Ed | 9.4 ± 0.0 Ed | 82.5 ± 0.0 Aa | 45.9 ± 0.1 Aa | 44.2 ± 0.1 Cb |
| ATP-1 | 8.9 ± 0.1 Dd | 10.2 ± 0.1 Cd | 80.9 ± 0.1 Ba | 46.0 ± 1.2 Aa | 44.7 ± 0.1 Bb | |
| ATP-2 | 9.1 ± 0.0 Cd | 9.9 ± 0.0 Dd | 81.0 ± 0.1 Ba | 46.2 ± 1.9 Ba | 45.0 ± 0.1 Bb | |
| ATP-3 | 12.4 ± 0.0 Ad | 13.4 ± 0.0 Ad | 74.2 ± 0.0 Da | 46.3 ± 1.1 Ba | 45.8 ± 0.1 Ab | |
| ATP-4 | 13.0 ± 0.1 Bd | 11.5 ± 0.1 Bd | 75.5 ± 0.1 Ca | 46.2 ± 2.5 Ba | 46.1 ± 0.4 Ac |
| Soil Types | Treatments | Van Genuchten | |||||
|---|---|---|---|---|---|---|---|
| θr | θs | A | n | R2 | RMSE | ||
| LS | CK | 0.077 | 0.480 | 0.039 | 1.317 | 0.9989 | 0.009 |
| ATP-1 | 0.082 | 0.496 | 0.040 | 1.310 | 0.9999 | 0.008 | |
| ATP-2 | 0.092 | 0.503 | 0.041 | 1.307 | 0.9999 | 0.006 | |
| ATP-3 | 0.093 | 0.503 | 0.035 | 1.305 | 0.9997 | 0.015 | |
| ATP-4 | 0.097 | 0.514 | 0.035 | 1.294 | 0.9999 | 0.008 | |
| DS | CK | 0.049 | 0.467 | 0.028 | 1.341 | 0.9995 | 0.005 |
| ATP-1 | 0.052 | 0.471 | 0.029 | 1.341 | 0.9999 | 0.005 | |
| ATP-2 | 0.052 | 0.480 | 0.029 | 1.338 | 0.9992 | 0.005 | |
| ATP-3 | 0.059 | 0.490 | 0.026 | 1.335 | 0.9996 | 0.008 | |
| ATP-4 | 0.060 | 0.493 | 0.025 | 1.316 | 0.9992 | 0.004 | |
| CS | CK | 0.061 | 0.448 | 0.023 | 1.520 | 0.9999 | 0.013 |
| ATP-1 | 0.062 | 0.460 | 0.024 | 1.497 | 0.9999 | 0.010 | |
| ATP-2 | 0.062 | 0.469 | 0.024 | 1.437 | 0.9998 | 0.007 | |
| ATP-3 | 0.067 | 0.476 | 0.025 | 1.425 | 0.9996 | 0.013 | |
| ATP-4 | 0.071 | 0.455 | 0.031 | 1.411 | 0.9999 | 0.007 | |
| SS | CK | 0.027 | 0.373 | 0.022 | 1.770 | 0.9996 | 0.004 |
| ATP-1 | 0.031 | 0.375 | 0.024 | 1.716 | 0.9999 | 0.005 | |
| ATP-2 | 0.041 | 0.383 | 0.029 | 1.702 | 0.9993 | 0.010 | |
| ATP-3 | 0.053 | 0.409 | 0.031 | 1.687 | 0.9999 | 0.008 | |
| ATP-4 | 0.057 | 0.486 | 0.032 | 1.649 | 0.9999 | 0.006 | |
| Soil Types | Treatments | FC (cm·cm−3) | PWP (cm·cm−3) | AWC (cm·cm−3) |
|---|---|---|---|---|
| LS | CK | 0.258 ± 0.008 Da | 0.133 ± 0.004 Da | 0.125 ± 0.004 Cb |
| ATP-1 | 0.267 ± 0.008 Ca | 0.139 ± 0.004 Ca | 0.128 ± 0.004 Bb | |
| ATP-2 | 0.276 ± 0.008 Ba | 0.148 ± 0.004 Ba | 0.128 ± 0.004 Bb | |
| ATP-3 | 0.281 ± 0.008 Ba | 0.153 ± 0.005 Ba | 0.128 ± 0.004 Bc | |
| ATP-4 | 0.299 ± 0.009 Aa | 0.163 ± 0.005 Aa | 0.136 ± 0.004 Cb | |
| DS | CK | 0.249 ± 0.012 Ca | 0.102 ± 0.005 Cb | 0.147 ± 0.007 Ba |
| ATP-1 | 0.252 ± 0.013 BCa | 0.105 ± 0.005 Bb | 0.146 ± 0.007 Ba | |
| ATP-2 | 0.255 ± 0.013 Bb | 0.107 ± 0.005 Bb | 0.148 ± 0.007 Ba | |
| ATP-3 | 0.273 ± 0.014 Aa | 0.117 ± 0.006 Ab | 0.156 ± 0.008 Aa | |
| ATP-4 | 0.272 ± 0.014 Ab | 0.116 ± 0.006 Ab | 0.156 ± 0.008 Aa | |
| CS | CK | 0.198 ± 0.008 Eb | 0.079 ± 0.003 Dc | 0.119 ± 0.005 Db |
| ATP-1 | 0.206 ± 0.008 Db | 0.082 ± 0.003 Cc | 0.124 ± 0.005 Cb | |
| ATP-2 | 0.216 ± 0.009 Cc | 0.086 ± 0.003 Bc | 0.131 ± 0.005 Bb | |
| ATP-3 | 0.229 ± 0.009 Bc | 0.086 ± 0.003 Bc | 0.143 ± 0.006 Aa | |
| ATP-4 | 0.234 ± 0.009 Ab | 0.091 ± 0.004 Ac | 0.143 ± 0.006 Ab | |
| SS | CK | 0.111 ± 0.010 Ec | 0.029 ± 0.003 Ed | 0.082 ± 0.007 Cc |
| ATP-1 | 0.122 ± 0.011 Dc | 0.033 ± 0.003 Dd | 0.088 ± 0.008 Bc | |
| ATP-2 | 0.140 ± 0.013 Cd | 0.044 ± 0.004 Cd | 0.096 ± 0.009 Ac | |
| ATP-3 | 0.154 ± 0.014 Bc | 0.057 ± 0.005 Bd | 0.097 ± 0.009 Ad | |
| ATP-4 | 0.161 ± 0.014 Ad | 0.065 ± 0.006 Ad | 0.096 ± 0.009 Ac |
| Soil Types | Treatments | WR0.25 (%) | W−MWD (mm) | W−GWD (mm) | DR0.25 (%) | D−MWD (mm) | D−GWD (mm) | PAD0.25 (%) |
|---|---|---|---|---|---|---|---|---|
| LS | CK | 29.6 ± 0.4 Db | 0.392 ± 0.014 Db | 0.243 ± 0.003 Db | 88.9 ± 0.4 Da | 3.244 ± 0.062 Da | 1.941 ± 0.050 Da | 66.7 ± 0.6 Ab |
| ATP-1 | 56.7 ± 0.2 Aa | 0.625 ± 0.012 Ca | 0.406 ± 0.003 Bb | 89.9 ± 0.2 Ca | 3.488 ± 0.055 Ca | 2.137 ± 0.046 Ca | 36.9 ± 0.3 Dc | |
| ATP-2 | 55.0 ± 0.2 Ba | 0.658 ± 0.023 Ba | 0.471 ± 0.007 Aa | 92.5 ± 0.2 Ba | 3.858 ± 0.046 Aa | 2.716 ± 0.052 Aa | 35.2 ± 0.4 Ec | |
| ATP-3 | 43.5 ± 0.8 Cc | 0.705 ± 0.034 Aa | 0.334 ± 0.010 Cc | 92.3 ± 0.6 Ba | 3.577 ± 0.065 BCa | 2.557 ± 0.088 Ba | 52.8 ± 1.2 Bb | |
| ATP-4 | 56.2 ± 0.3 Ba | 0.596 ± 0.022 Cb | 0.413 ± 0.006 Bc | 93.5 ± 0.4 Aa | 3.642 ± 0.051 Ba | 2.676 ± 0.065 Aa | 39.9 ± 0.6 Cd | |
| DS | CK | 33.7 ± 0.8 Dd | 0.501 ± 0.027 Ca | 0.216 ± 0.006 Ec | 81.4 ± 0.2 Eb | 3.196 ± 0.058 Ca | 1.709 ± 0.040 Db | 58.5 ± 1.0 Ac |
| ATP-1 | 36.7 ± 2.4 Cb | 0.565 ± 0.064 Ba | 0.257 ± 0.003 Dc | 84.1 ± 0.3 Db | 3.353 ± 0.056 Bb | 1.974 ± 0.041 Cb | 56.4 ± 2.9 Ab | |
| ATP-2 | 52.6 ± 0.4 Bb | 0.580 ± 0.026 Bb | 0.418 ± 0.007 Cb | 85.2 ± 0.3 Cb | 3.416 ± 0.055 Bb | 2.082 ± 0.049 Bb | 38.2 ± 0.7 Cb | |
| ATP-3 | 62.7 ± 0.2 Aa | 0.675 ± 0.022 Aa | 0.516 ± 0.006 Bb | 87.7 ± 0.2 Ab | 3.523 ± 0.043 Aa | 2.335 ± 0.041 Ab | 28.6 ± 0.3 Dc | |
| ATP-4 | 51.0 ± 0.2 Bb | 0.714 ± 0.011 Aa | 0.550 ± 0.003 Aa | 86.7 ± 0.3 Bb | 3.583 ± 0.052 Aa | 2.397 ± 0.059 Ab | 41.2 ± 0.5 Bc | |
| CS | CK | 18.1 ± 0.5 Dd | 0.271 ± 0.016 Bc | 0.155 ± 0.003 Cd | 74.0 ± 0.0 Ec | 2.957 ± 0.037 Db | 1.536 ± 0.023 Ec | 75.6 ± 0.7 Ba |
| ATP-1 | 19.6 ± 0.8 Cd | 0.317 ± 0.024 Ac | 0.183 ± 0.005 Bd | 75.4 ± 0.1 Cc | 3.061 ± 0.035 Cc | 1.664 ± 0.024 Cc | 74.0 ± 1.1 Ba | |
| ATP-2 | 32.8 ± 0.5 Ad | 0.336 ± 0.018 Ad | 0.244 ± 0.004 Ac | 76.4 ± 0.1 Bc | 3.289 ± 0.034 Ac | 1.836 ± 0.026 Bc | 57.1 ± 0.6 Da | |
| ATP-3 | 17.8 ± 0.8 Dd | 0.348 ± 0.022 Ac | 0.186 ± 0.005 Bd | 78.7 ± 0.12 Ac | 3.209 ± 0.037 Bb | 1.960 ± 0.033 Ac | 77.4 ± 1.1 Aa | |
| ATP-4 | 22.3 ± 0.8 Bd | 0.329 ± 0.026 Ac | 0.161 ± 0.005 Cd | 74.9 ± 0.0 Dc | 3.030 ± 0.041 Cb | 1.603 ± 0.026 Dc | 70.2 ± 1.1 Ca | |
| SS | CK | 23.6 ± 1.9 Ec | 0.315 ± 0.061 Bc | 0.368 ± 0.014 Ea | 30.1 ± 0.8 Ed | 0.788 ± 0.089 Dc | 0.567 ± 0.019 Ed | 21.7 ± 4.2 Cd |
| ATP-1 | 33.1 ± 1.2 Dc | 0.432 ± 0.042 Ab | 0.514 ± 0.009 Ba | 46.1 ± 0.8 Dd | 1.465 ± 0.079 Cd | 0.753 ± 0.023 Dd | 28.2 ± 1.3 Bd | |
| ATP-2 | 44.1 ± 0.5 Bc | 0.409 ± 0.026 Ac | 0.480 ± 0.006 Ca | 48.7 ± 0.4 Cd | 1.553 ± 0.079 Cd | 0.851 ± 0.025 Cd | 9.5 ± 0.3 Ed | |
| ATP-3 | 48.8 ± 0.5 Ab | 0.434 ± 0.033 Ab | 0.554 ± 0.021 Aa | 56.7 ± 0.6 Bd | 2.082 ± 0.064 Bc | 1.077 ± 0.027 Bd | 14.0 ± 0.1 Dd | |
| ATP-4 | 37.4 ± 0.7 Cc | 0.318 ± 0.030 Bc | 0.428 ± 0.007 Db | 66.3 ± 1.3 Ad | 2.557 ± 0.057 Ac | 1.437 ± 0.039 Ad | 43.6 ± 0.0 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Xing, X.; Gao, Y.; Ma, X. An Environmentally Friendly Soil Amendment for Enhancing Soil Water Availability in Drought-Prone Soils. Agronomy 2022, 12, 133. https://doi.org/10.3390/agronomy12010133
Yang T, Xing X, Gao Y, Ma X. An Environmentally Friendly Soil Amendment for Enhancing Soil Water Availability in Drought-Prone Soils. Agronomy. 2022; 12(1):133. https://doi.org/10.3390/agronomy12010133
Chicago/Turabian StyleYang, Ting, Xuguang Xing, Yan Gao, and Xiaoyi Ma. 2022. "An Environmentally Friendly Soil Amendment for Enhancing Soil Water Availability in Drought-Prone Soils" Agronomy 12, no. 1: 133. https://doi.org/10.3390/agronomy12010133
APA StyleYang, T., Xing, X., Gao, Y., & Ma, X. (2022). An Environmentally Friendly Soil Amendment for Enhancing Soil Water Availability in Drought-Prone Soils. Agronomy, 12(1), 133. https://doi.org/10.3390/agronomy12010133