Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Bioassay Procedure
2.3. Separation of the Active Fraction of the Aqueous Methanol Extract from the S. garrettiana Leaves
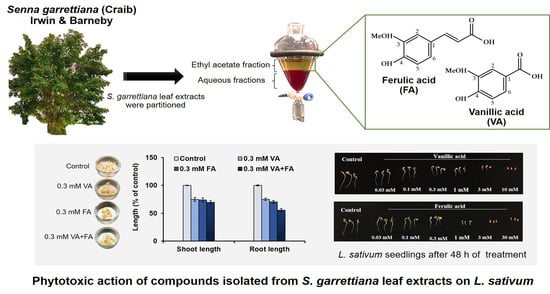
2.4. Isolation and Identification of the Phytotoxic Substances in the S. garrettiana Leaves
2.5. Bioassay of the Phytotoxic Substances from the S. garrettiana Leaves
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biological Activity of the the Aqueous Methanol Extracts of S. garrettiana Leaf
3.2. Separation of the Active Fraction in the S. garrettiana Leaf Extracts
3.3. Isolation and Identification of the Phytotoxic Substances in the S. garrettiana Leaf Extracts
3.4. Biological Activity of the Compounds Isolated from the S. garrettiana Leaf Extract against L. sativum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 67–68. [Google Scholar]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Chu, C.; Mortimer, P.E.; Wang, H.; Wang, Y.; Liu, X.; Yu, S. Allelopathic effects of Eucalyptus on native and introduced tree species. For. Ecol. Manag. 2014, 323, 79–84. [Google Scholar] [CrossRef]
- Macías, F.A.; Marin, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Kato-Noguchi, H.; Fushimi, Y.; Shigemori, H. An allelopathic substance in red pine needles (Pinus densiflora). J. Plant Physiol. 2009, 166, 442–446. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Chapter two—Allelopathy and the role of allelochemicals in plant defence. Adv. Bot. Res. 2017, 82, 19–54. [Google Scholar] [CrossRef]
- Peres, M.T.L.P.; da Silva Cândido, A.C.; Bonilla, M.B.; Faccenda, O.; Hess, S.C. Phytotoxic potential of Senna occidentalis and Senna obtusifolia. Acta Sci. Biol. Sci. 2010, 32, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, I.F.; Vieira, E.A. Phytotoxic potential of Senna occidentalis (L.) Link extracts on seed germination and oxidative stress of Ipê seedlings. Plant Biol. 2019, 21, 770–779. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- El-Deek, M.H.; Hess, F.D. Inhibited mitotic entry is the cause of growth inhibition by cinmethylin. Weed Sci. 1986, 34, 684–688. [Google Scholar] [CrossRef]
- Grossmann, K.; Hutzler, J.; Tresch, S.; Christiansen, N.; Looser, R.; Ehrhardt, T. On the mode of action of the herbicides cinmethylin and 5-benzyloxymethyl-1, 2-isoxazolines: Putative inhibitors of plant tyrosine aminotransferase. Pest Manag. Sci. 2011, 68, 482–492. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of three potential phytotoxic compounds from Chrysopogon aciculatus (Retz.) Trin. Acta Physiol. Plant. 2021, 43, 56. [Google Scholar] [CrossRef]
- Alsaadawi, I.; Khaliq, A.; Al-Temimi, A.; Matloob, A. Integration of sunflower (Helianthus annuus) residues with a pre-plant herbicide enhances weed suppression in broad bean (Vicia faba). Planta Daninha 2011, 29, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Poonpaiboonpipat, T.; Krumsri, R.; Kato-Noguchi, H. Allelopathic and herbicidal effects of crude extract from Chromolaena odorata (L.) R.M. King and H. Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Lewis, G.P. Tribe Cassieae. In Legumes of the World; Lewis, G.P., Scrhir, B., Mackinder, B., Lock, M., Eds.; Royal Botanic Garden: Kew, UK, 2005; pp. 111–125. [Google Scholar]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Islam, M.S. Anti-diabetic effects of the acetone fraction of Senna singueana stem bark in a type 2 diabetes rat model. J. Ethnopharmacol. 2014, 153, 392–399. [Google Scholar] [CrossRef]
- Torquato, I.H.S.; da Costa, N.C.; Pereira, K.S.; Campos, N.B.; de Oliveira, A.A.; Generino, M.E.M.; Bezerra, J.W.A.; dos Santos, M.A.F.; Sousa, J.D.; Boligon, A.A.; et al. Polyphenolic composition and allelopathic potential of Senna cearensis Afr. fern. (Fabaceae). Res. Soc. Dev. 2020, 9, e577986207. [Google Scholar] [CrossRef]
- Monkheang, P.; Sudmoon, R.; Tanee, T.; Noikotr, K.; Bletter, N.; Chaveerach, A. Species diversity, usages, molecular markers and barcode of medicinal Senna species (Fabaceae, Caesalpinioideae) in Thailand. J. Med. Plant Res. 2011, 5, 6173–6181. [Google Scholar] [CrossRef]
- Pattarapongdilok, N.; Malichim, P.; Simmee, N.; Sichaem, J. Senna flower extract as an indicator for acid-base titration. Rasayan J. Chem. 2021, 14, 1402–1407. [Google Scholar] [CrossRef]
- Surapanthanakorn, S.; Phadoongsombut, N.; Wattanapiromsakul, C.; Reanmongkol, W. In vivo evaluation of analgesic and antipyretic activities of piceatannol-rich extract from Senna garrettiana heartwood. Songklanakarin J. Sci. Technol. 2016, 39, 589–599. [Google Scholar]
- Sakunpak, A.; Saingam, W. Screening and compound isolation from selected Thai herbal medicine for anti-hyaluronidase and anti-elastase activities. In Proceedings of the RSU International Research Conference (2020), Bangkok, Thailand, 1 May 2020; pp. 305–311. [Google Scholar]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Surapanthanakorn, S.; Wattanapiromsakul, C.; Reanmongkol, W. Assessment of the anti-inflammatory activity of piceatannol-rich extract from Senna garrettiana heartwood. Chiang Mai J. Sci. 2018, 45, 2691–2702. [Google Scholar]
- Baez, D.A.; Zepeda Vallejo, L.G.; Jimenez-Estrada, M. Phytochemical studies on Senna skinneri and Senna wislizeni. Nat. Prod. Lett. 1999, 13, 223–228. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Franco, D.M.; Silva, E.M.; Saldanha, L.L.; Adachi, S.A.; Schley, T.R.; Rodrigues, T.M.; Dokkedal, A.L.; Nogueira, F.T.S.; Rolim de Almeida, L.F. Flavonoids modify root growth and modulate expression of SHORT-ROOT and HD-ZIP III. J. Plant Physiol. 2015, 188, 89–95. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Boonmee, S.; Suwitchayanon, P.; Krumsri, R.; Kato-Noguchi, H. Investigation of the allelopathic potential of Nephrolepis cordifolia (L.) C. Presl against dicotyledonous and monocotyledonous plant species. Environ. Control. Biol. 2020, 58, 71–78. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Dalbergia cochinchinensis Pierre and its growth inhibitory substance. Emir. J. Food Agric. 2020, 32, 513–521. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.; Asib, N.B.; Islam, A.K.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical constituents and allelopathic potential of Parthenium hysterophorus L. in comparison to commercial herbicides to control weeds. Plants 2021, 10, 1445. [Google Scholar] [CrossRef]
- Rob, M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a novel compound involved in allelopathic activity of Garcinia Xanthochymus hook. Plants 2019, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Krumsri, R.; Kato-Noguchi, H.; Poonpaiboonpipat, T. Allelopathic effect of Sphenoclea zeylanica Gaertn. on rice (Oryza sativa L.) germination and seedling growth. Aust. J. Crop Sci. 2020, 14, 1450–1455. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Kato-Noguchi, H. Assessment of allelopathic activity of Tradescantia spathacea Sw. for weed control. Biol. Futur. 2021, 1–7. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.B.; Candido, A.C.S.; Simionatto, E.; Faccenda, O.; Scalon, S.D.P.Q.; Peres, M.T.L.P. Allelopathic and antioxidant activity and total phenolic contents of Hydrocotyle bonariensis Lam. (Araliaceae)/Atividade alelopatica, antioxidante e teor de fenois totais de Hydrocotyle bonariensis Lam. (Araliaceae). Acta Sci. Technol. 2010, 32, 413–421. [Google Scholar]
- Pereira, J.C.; Paulino, C.; Endres, L.; Santana, A.E.G.; Pereira, F.R.S.; Souza, R.C. Allelopathic potential of ethanolic extract and phytochemical analysis of Paspalum maritimum Trind. Planta Daninha 2019, 37, 1–12. [Google Scholar] [CrossRef]
- Blum, U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 28, 259–267. [Google Scholar] [PubMed]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of allelopathic interaction of essential oils from medicinal and aromatic plants on seed germination and seedling growth of lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, D.U.; Lee, K.R. A new phenylpropane glycoside from the rhizome of Sparganium stoloniferum. Arch. Pharm. Res. 2010, 33, 515–521. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. J. Nutr. 2016, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampietro, D.A.; Vattuone, M.A.; Isla, M.I. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) straw. J. Plant Physiol. 2006, 63, 837–846. [Google Scholar] [CrossRef]
- Hussain, F.; Ghulam, S.; Sher, Z.; Ahmad, B. Allelopathy by Lantana camara. Pak. J. Bot. 2011, 43, 2373–2378. [Google Scholar]
- Gurmani, A.R.; Khan, S.U.; Mehmood, T.; Ahmed, W.; Rafique, M. Exploring the allelopathic potential of plant extracts for weed suppression and productivity in wheat (Triticum aestivum L.). Gesunde Pflanz. 2021, 73, 29–37. [Google Scholar] [CrossRef]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tanga, Z.; Liub, M.; Fua, C.; Zhangc, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ahmad, S.; Ajaz Rasool, S.; Asad Sayeed, S.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Aljović, I.; Gojak-Salimović, S. Evaluation of the antioxidant activity of ferulic, homovanillic and vanillic acids using the Briggs-Rauscher oscillating reaction method. Glas. Hem. Tehnol. Bosne Herceg. 2017, 49, 35–38. [Google Scholar]
- Li, Q.; Chang, X.; Guo, R.; Wang, Q.; Guo, X. Dynamic effects of fermentation on phytochemical composition and antioxidant properties of wampee (Clausena lansium (Lour.) Skeel) leaves. Food Sci. Nutr. 2019, 7, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Inderjit; Nilsen, E.T. Bioassays and field studies for allelopathy in terrestrial plants: Progress and problems. Crit. Rev. Plant Sci. 2003, 22, 221–238. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Rasmussen, J.A. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 1979, 5, 815–824. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, K.H.; Ahn, J.K.; Chun, S.C.; Kim, C.S.; Kim, J.T.; Kim, S.H. Screening of allelochemicals on barnyardgrass (Echinochloa crus-galli) and identification of potentially allelopathic compounds from rice (Oryza sativa) variety hull extracts. J. Crop Prot. 2002, 21, 913–920. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, J.; Luan, G.; Zhang, S.; Zhuoma, Y.; Xie, J.; Zhou, W. Quantitative analyses of nine phenolic compounds and their antioxidant activities from thirty-seven varieties of raspberry grown in the qinghai-tibetan plateau region. Molecules 2019, 24, 3932. [Google Scholar] [CrossRef] [Green Version]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Poonpaiboonpipat, T.; Pangnakorn, U.; Suvunnamek, U.; Teerarak, M.; Charoenying, P.; Laosinwattana, C. Phytotoxic effects of essential oil from Cymbopogon citratus and its physiological mechanisms on barnyardgrass (Echinochloa crus-galli). Ind. Crops Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Ren, K.; Cheng, Z. Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Mecina, G.F.; Chia, M.A.; Cordeiro-Araújo, M.K.; do Carmo Bittencourt-Oliveira, M.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Macías, F.A.; da Silva, R.M.G. Effect of flavonoids isolated from Tridax procumbens on the growth and toxin production of Microcystis aeruginos. Aquat. Toxicol. 2019, 211, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D. Allelopathic effects of Lantana camara L. on Lathyrus sativus L.: Oxidative imbalance and cytogenetic consequences. Allelopathy J. 2013, 31, 71–90. [Google Scholar]
- Rial, C.; García, B.F.; Varela, R.M.; Torres, A.; Molinillo, J.M.; Macías, F.A. The joint action of sesquiterpene lactones from leaves as an explanation for the activity of Cynara cardunculus. J. Agric. Food Chem. 2016, 64, 6416–6424. [Google Scholar] [CrossRef] [PubMed]
- Tena, C.; Santiago, A.D.R.; Osuna, D.; Sosa, T. Phytotoxic activity of p-cresol, 2-phenylethanol and 3-phenyl-1-propanol, phenolic compounds present in Cistus ladanifer L. Plants 2021, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint action of phenolic acid mixtures and its significance in allelopathy research. Physiol. Plant. 2002, 114, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Kudsk, P.; Mathiassen, S.K. Joint action of benzoxazinone derivatives and phenolic acids. J. Agric. Food Chem. 2006, 54, 1049–1057. [Google Scholar] [CrossRef] [PubMed]





| Test Plant Species | Leaf Extract Concentration (mg DW Equivalent Extract mL−1) | Shoot Length (mm) | Root Length (mm) |
|---|---|---|---|
| L. sativum | Control | 8.47 ± 0.27 a | 17.50 ± 0.97 a |
| 1 | 8.12 ± 0.30 a | 17.27 ± 0.71 a | |
| 3 | 7.24 ± 0.33 b | 15.87 ± 0.57 a | |
| 10 | 5.10 ± 0.22 c | 11.10 ± 2.22 b | |
| 30 | 1.08 ± 0.14 d | 2.22 ± 0.13 c | |
| 100 | 1.25 ± 0.09 e | 1.07 ± 0.04 cd | |
| 300 | 0.00 ± 0.00 f | 0.00 ± 0.00 d | |
| F | 3.05 | 24.58 | |
| p-value | <0.001 | <0.001 | |
| E. crus-galli | Control | 19.73 ± 1.02 a | 15.43 ± 0.99 a |
| 1 | 18.60 ± 0.52 a | 15.33 ± 0.46 a | |
| 3 | 15.55 ± 0.66 b | 12.13 ± 0.55 b | |
| 10 | 13.61 ± 0.58 b | 9.27 ± 0.57 c | |
| 30 | 10.35 ± 0.69 c | 4.48 ± 0.52 d | |
| 100 | 3.95 ± 0.18 d | 1.35 ± 0.12 e | |
| 300 | 0.00 ± 0.00 e | 0.00 ± 0.00 e | |
| F | 154.86 | 1.79 | |
| p-value | <0.001 | <0.001 | |
| Interaction species × treatment | F | 53.85 | 85.52 |
| p-value | <0.001 | <0.001 |
| Fraction | IC50 Value (mg DW Equivalent Extract mL−1) | |||
|---|---|---|---|---|
| L. sativum | E. crus-galli | |||
| Shoot | Root | Shoot | Root | |
| Aqueous | 21.93 | 18.12 | 26.23 | 22.40 |
| Ethyl acetate | 16.13 | 13.32 | 19.87 | 18.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances. Agronomy 2022, 12, 139. https://doi.org/10.3390/agronomy12010139
Krumsri R, Iwasaki A, Suenaga K, Kato-Noguchi H. Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances. Agronomy. 2022; 12(1):139. https://doi.org/10.3390/agronomy12010139
Chicago/Turabian StyleKrumsri, Ramida, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2022. "Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances" Agronomy 12, no. 1: 139. https://doi.org/10.3390/agronomy12010139
APA StyleKrumsri, R., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2022). Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances. Agronomy, 12(1), 139. https://doi.org/10.3390/agronomy12010139