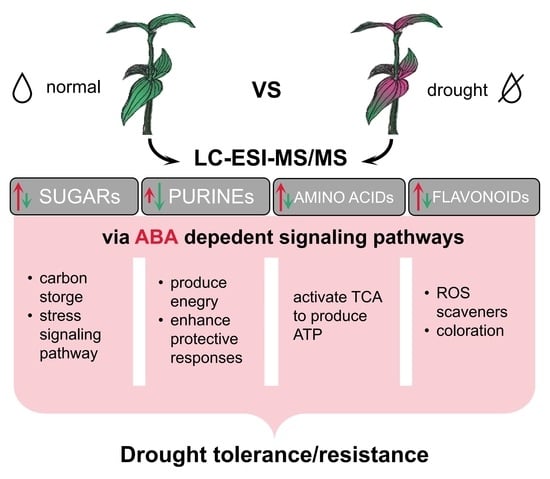
Metabolomic Response to Drought Stress in Belosynapsis ciliata (Blume) ‘Qiuhong’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Estimation of Physiological Traits
2.3. Sample Preparation and Metabolite Extraction
2.4. ESI-Q TRAP-MS/MS Analysis
2.5. Metabolite Identification and Quantification
3. Results
3.1. Physiochemical Changes in B. ciliata under Drought Treatment
3.2. Characterisation of Drought-Responsive Metabolites in B. ciliata
3.3. Multivariate Analysis of Metabolites Related to Drought Tolerance in ‘Qiuhong’
3.4. Global Metabolic Reprogramming Induced by Drought Treatments in ‘Qiuhong’
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA: | Abscisic Acid |
| ATP: | Adenosine Triphosphate |
| CAD: | Collision Activated Dissociation |
| CAT: | Catalase Activity |
| CE: | Collision Energy |
| CoP: | p-Coumaroyl Putrescine |
| CUR: | Curtain Gas |
| DAMs: | Differentially Accumulated Metabolites |
| DP: | Declustering Potential |
| ESI: | Electrospray Ionization |
| GS I: | Gas I |
| GS II: | Gas II |
| HPLC: | High-Performance Liquid Chromatography |
| IS: | Ion Spray Voltage |
| KEGG: | Kyoto Encyclopedia of Genes and Genomes |
| LC-ESI-MS/MS: | Liquid Chromatography Coupled with ESI-Triple Quadrupole-Linear Ion Trap Mass Spectrometry |
| LIT: | Linear Ion Trap |
| MC: | Moisture Content |
| MDA: | Malondialdehyde |
| MRM: | Multiple Reaction Monitoring |
| MS: | Mass Spectrometry |
| NAD+: | Nicotinamide Adenine Dinucleotide |
| NBT: | Nitroblue Tetrazolium |
| PA: | Phenolamide |
| PCA: | Principal Component Analysis |
| PLS-DA: | Partial Least Squares-Discriminant Analysis |
| POD: | Peroxidase Activity |
| QQQ: | Triple Quadrupole |
| RH: | Rehydration |
| ROS: | Reactive Oxygen Species |
| SMs: | Secondary Metabolites |
| SOD: | Superoxide Dismutase |
| SPSS: | Statistical Product and Service Solutions |
| TCA: | Citrate Cycle |
| UV: | Ultraviolet |
| VIP: | Variable Importance in Projection |
References
- McKay, J.K.; Richards, J.H.; Mitchell-Olds, T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol. Ecol. 2003, 12, 1137–1151. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Taiz, L.Z.E. Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Verma, A.; Deepti, S. Abiotic stress and crop improvement: Current scenario. Adv. Plants Agric. Res. 2016, 4, 00149. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. Int. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Lal, S.K.; Pawar, J.; Narayan, O.P. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 2021, 25, 100264. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Asgher, M.; Fatma, M.; Per, T.S.; Khan, N.A. Drought stress vis a vis plant functions in the era of climate change. Clim. Change Environ. Sustain. 2015, 3, 13–25. [Google Scholar] [CrossRef]
- Tardieu, F.; Parent, B.; Caldeira, C.F.; Welcker, C. Genetic and physiological controls of growth under water deficit. Plant Physiol. 2014, 164, 1628–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Xu, Y.; Wu, W.; Ye, Y.; Chen, P. The complete chloroplast genome sequence of Belosynapsis ciliata (Blume) R. S. Rao (Commelinaceae). Mitochondrial DNA Part B Resour. 2019, 4, 2373–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, D.; DeFilipps, R.A. Commelinaceae. In Flora of China; Wu, Z., Raven, P.H., Hong, D., Eds.; Science Press, Beijing and Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 19–39. [Google Scholar]
- Xu, Y.; Yu, J.; Deng, L.; Guo, W.; Liu, P.; Ping, C.; Hong, L.; Hu, Z. Heat resistance research by Logisitic equation on four species of roof greening in Commelinaceae (In Chinese). J. Zhongkai Univ. Agric. Eng. 2018, 31, 52–55. [Google Scholar] [CrossRef]
- Yang, F.; Yang, T.; Liu, K.; Yang, Q.; Wan, Y.; Wang, R.; Li, G. Analysis of metabolite accumulation related to pod color variation of Caragana intermedia. Molecules 2019, 24, 717. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhou, R.; Xu, K.; Xu, J.; Jin, J.; Fang, H.; He, Y. Rapid determination of chlorophyll and pheophytin in green tea using fourier transform infrared spectroscopy. Molecules 2018, 23, 1010. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, F.; Tanaka, M.; Maeda, H.; Shimizu, K.; Sakata, Y. Characterization of cyanic flower color of delphinium cultivars. J. Jpn. Soc. Hortic. Sci. 2000, 69, 428–434. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Alici, E.H.; Arabaci, G. Determination of SOD, POD, PPO and CAT enzyme activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Wang, Q.; Su, Z.; Zhang, S.; Li, Y. Soluble sugar content of clonal plant Neosinocalamus affinis at module and ramet levels. J. Appl. Ecol. 2004, 15, 1994–1998. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Yuan, H.; Zeng, X.; Shi, J.; Xu, Q.; Wang, Y.; Jabu, D.; Sang, Z.; Nyima, T. Time-course comparative metabolite profiling under osmotic stress in tolerant and sensitive Tibetan hulless barley. Biomed. Res. Int. 2018, 2018, 9415409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, E.; Holton, K.; Nair, S.; Schlauch, D.; Sinha, R.; Quackenbush, J. MeV: MultiExperiment Viewer. In Biomedical Informatics for Cancer Research; Ochs, M.F., Casagrande, J.T., Davuluri, R.V., Eds.; Springer US: Boston, MA, USA, 2010; pp. 267–277. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Klíma, M.; Roy, A.; Prášil, I.T. Biological networks underlying abiotic stress tolerance in temperate crops—A proteomic perspective. Int. J. Mol. Sci. 2015, 16, 20913–20942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef] [Green Version]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Galili, G. The aspartate-family pathway of plants: Linking production of essential amino acids with energy and stress regulation. Plant Signal. Behav. 2011, 6, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, D.; Li, M.; Shi, L. Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PLoS ONE 2016, 11, e0159622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baena-González, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Minh, L.T.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z.; et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 2010, 61, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Stasolla, C.; Katahira, R.; Thorpe, T.A.; Ashihara, H. Purine and pyrimidine nucleotide metabolism in higher plants. J. Plant Physiol. 2003, 160, 1271–1295. [Google Scholar] [CrossRef]
- Watanabe, S.; Kounosu, Y.; Shimada, H.; Sakamoto, A. Arabidopsis xanthine dehydrogenase mutants defective in purine degradation show a compromised protective response to drought and oxidative stress. Plant Biotechnol. 2014, 31, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Matsumoto, M.; Hakomori, Y.; Takagi, H.; Shimada, H.; Sakamoto, A. The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ. 2014, 37, 1022–1036. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.C.; Jin, J.Q.; Ma, J.Q.; Yao, M.Z.; Ma, C.L.; Li, C.F.; Ding, Z.T.; Chen, L. Transcriptomic analysis of tea plant responding to drought stress and recovery. PLoS ONE 2016, 11, e0147306. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant. Sci. 2020, 11. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Back, K.; Jang, S.M.; Lee, B.C.; Schmidt, A.; Strack, D.; Kim, K.M. Cloning and characterization of a hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase induced in response to UV-C and wounding from Capsicum annuum. Plant Cell Physiol. 2001, 42, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izaguirre, M.M.; Mazza, C.A.; Svatos, A.; Baldwin, I.T.; Ballaré, C.L. Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Ann. Bot. 2007, 99, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Heinzel, N.; Schöttner, M.; Baldwin, I.T.; Gális, I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol. 2010, 152, 1731–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Tanguy, J. The occurrence and possible function of hydroxycinnamoyl acid amides in plants. Plant Growth Regul. 1985, 3, 381–399. [Google Scholar] [CrossRef]
- Stoessl, A.; Unwin, C.H. The antifungal factors in barley. V. antifungal activity of the hordatines. Can. J. Bot. 1970, 48, 465–470. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Poddar, S.K.; Sifat, A.E.; Haque, S.; Nahid, N.A.; Chowdhury, S.; Mehedi, I. Nicotinamide mononucleotide: Exploration of diverse therapeutic applications of a potential molecule. Biomolecules 2019, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S.I. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [Green Version]
- Stein, L.R.; Imai, S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef] [Green Version]
- Ueda, K.; Nakajima, Y.; Inoue, H.; Kobayashi, K.; Nishiuchi, T.; Kimura, M.; Yaeno, T. Nicotinamide mononucleotide potentiates resistance to biotrophic invasion of fungal pathogens in barley. Int. J. Mol. Sci. 2021, 22, 2696. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, B.; Huang, R.; Wen, M.; Huang, L.; Su, Y.; Sun, Y.; Wang, N.; Guo, W. Metabolomic Response to Drought Stress in Belosynapsis ciliata (Blume) ‘Qiuhong’. Agronomy 2022, 12, 466. https://doi.org/10.3390/agronomy12020466
Li Y, Zhang B, Huang R, Wen M, Huang L, Su Y, Sun Y, Wang N, Guo W. Metabolomic Response to Drought Stress in Belosynapsis ciliata (Blume) ‘Qiuhong’. Agronomy. 2022; 12(2):466. https://doi.org/10.3390/agronomy12020466
Chicago/Turabian StyleLi, Yongquan, Bipei Zhang, Runsheng Huang, Min Wen, Leying Huang, Yiting Su, Yanjun Sun, Ning Wang, and Wei Guo. 2022. "Metabolomic Response to Drought Stress in Belosynapsis ciliata (Blume) ‘Qiuhong’" Agronomy 12, no. 2: 466. https://doi.org/10.3390/agronomy12020466
APA StyleLi, Y., Zhang, B., Huang, R., Wen, M., Huang, L., Su, Y., Sun, Y., Wang, N., & Guo, W. (2022). Metabolomic Response to Drought Stress in Belosynapsis ciliata (Blume) ‘Qiuhong’. Agronomy, 12(2), 466. https://doi.org/10.3390/agronomy12020466