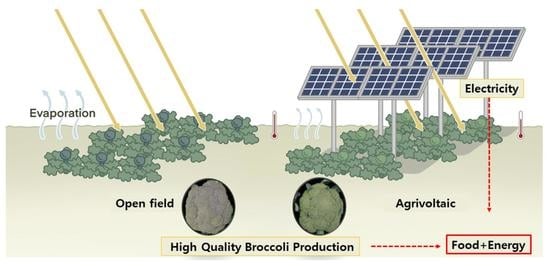
Agrivoltaic Systems Enhance Farmers’ Profits through Broccoli Visual Quality and Electricity Production without Dramatic Changes in Yield, Antioxidant Capacity, and Glucosinolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broccoli Cultivation under Agrivoltaic Systems and Shading Treatment
2.2. Plant Growth Parameters
2.3. Sample Extraction
2.4. Total Phenolic Content (TPC)
2.5. Total Flavonoid Content (TFC)
2.6. Determination of the Antioxidant Activity by the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free-Radical Scavenging Assay
2.7. Quantitation of Glucosinolate
2.8. Glucosinolate Hydrolysis Products
2.9. Color Characteristic Measurements
2.10. Microclimatological Measurements
2.11. Electricity Generation and Economic Evaluation of Solar Panel
2.12. Statistical Analysis
3. Results and Discussion
3.1. Plant Growth Environment
3.2. Characteristic of Growing Parameter
3.3. Antioxidant Compounds and Capacity (TPC, TFC, and DPPH)
3.4. Quantitation of Glucosinolates and Their Hydrolysis Products
3.5. Broccoli Visual Quality Change by AV Structure with Additional Shading
3.6. Economic Evaluation of Solar Panel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bizikova, L.; Dimple, R.; Swanson, D.; Venema, H.D.; McCandless, M. The Water-Energy-Food Security Nexus: Towards a Practical Planning and Decision-Support Framework for Landscape Investment and Risk Management; The International Institute for Sustainable Development: Winnipeg, MB, Canada, 2013. [Google Scholar]
- Slaymane, A.; Salim, R.; Soliman, M.R. Integrated Water Balance and Water Quality Management under Future Climate Change and Population Growth: A Case Study of Upper Litani Basin, Lebanon; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Dupraz, C.; Marrou, H.; Talbot, G.; Dufour, L.; Nogier, A.; Ferard, Y. Combining Solar Photovoltaic Panels and Food Crops for Optimising Land Use: Towards New Agrivoltaic Schemes. Renew. Energy 2011, 36, 2725–2732. [Google Scholar] [CrossRef]
- Sánchez-Friera, P.; Lalaguna, B.; Montiel, D.; Gil, J.; Caballero, L.J.; Alonso, J.; Piliougine, M.; Sidrach De Carmona, M. Development and Characterisation of Industrial Bifacial Pv Modules with Ultra-Thin Screen-Printed Solar Cells. In Proceedings of the 22nd European Photovoltaic Solar Energy Conference, Milan, Italy, 3–7 September 2007. [Google Scholar]
- Smith, E.L. Photosynthesis in Relation to Light and Carbon Dioxide. Proc. Natl. Acad. Sci. USA 1936, 22, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tazawa, S. Effects of Various Radiant Sources on Plant Growth (Part 1). Jpn. Agric. Res. Q. 1999, 33, 163–176. [Google Scholar]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.; Wallig, M.A.; Juvik, J.A. Carotene, Tocopherol, and Ascorbate Contents in Subspecies of Brassica Oleracea. J. Agric. Food Chem. 1999, 47, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Borowski, J.; Szajdek, A.; Borowska, E.J.; Ciska, E.; Zieliński, H. Content of selected bioactive components and antioxidant properties of broccoli (Brassica oleracea L.). Eur. Food Res. Technol. 2007, 226, 459–465. [Google Scholar] [CrossRef]
- Kohl, W.L. The International Energy Agency: The Political Context. In Oil, the Arab-Israel Dispute, and the Industrial World; Routledge: London, UK, 2019; pp. 246–257. [Google Scholar]
- Lampe, J.W.; Peterson, S. Brassica, Biotransformation and Cancer Risk: Genetic Polymorphisms Alter the Preventive Effects of Cruciferous Vegetables. J. Nutr. 2002, 132, 2991–2994. [Google Scholar] [CrossRef] [Green Version]
- Minich, D.M.; Bland, J.S. A Review of the Clinical Efficacy and Safety of Cruciferous Vegetable Phytochemicals. Nutr. Rev. 2007, 65, 259–267. [Google Scholar] [CrossRef]
- Murillo, G.; Mehta, R.G. Cruciferous Vegetables and Cancer Prevention. Nutr. Cancer 2001, 41, 17–28. [Google Scholar] [CrossRef]
- Topcu, Y.; Dogan, A.; Kasimoglu, Z.; Sahin-Nadeem, H.; Polat, E.; Erkan, M. The effects of UV radiation during the vegetative period on antioxidant compounds and postharvest quality of broccoli (Brassica oleracea L.). Plant Physiol. Biochem. 2015, 93, 56–65. [Google Scholar] [CrossRef]
- Garitta, L.; Hough, G.; Chaves, A. Sensory analysis of broccoli over time: Consumer defined critical attributes and evaluation of digital photographs in comparison to real product appearance. Food Qual. Prefer. 2013, 29, 48–52. [Google Scholar] [CrossRef]
- Coles, P. Produce Buyer Quality Requirements to Form an Eastern Broccoli Industry. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2016. [Google Scholar]
- Singh, R.; Kumar, S.; Kumar, S. Performance and Preference of Broccoli Varieties Grown under Low Hill Conditions of Himachal Pradesh. Indian Res. J. Ext. Educ. 2016, 14, 112–114. [Google Scholar]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics Analysis Reveals the Compositional Differences of Shade Grown Tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-M.; Jeffery, E.H.; Juvik, J.A.; Kushad, M.M. Correlation of Quinone Reductase Activity and Allyl Isothiocyanate Formation Among Different Genotypes and Grades of Horseradish Roots. J. Agric. Food Chem. 2015, 63, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic Control of Natural Variation in Arabidopsis Glucosinolate Accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Optimization of methyl jasmonate application to broccoli florets to enhance health-promoting phytochemical content. J. Sci. Food Agric. 2013, 94, 2090–2096. [Google Scholar] [CrossRef]
- Ku, K.-M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.-H.; Juvik, J.A. Profiles of Glucosinolates, Their Hydrolysis Products, and Quinone Reductase Inducing Activity from 39 Arugula (Eruca sativa Mill.) Accessions. J. Agric. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef]
- Kim, M.J.; Chiu, Y.-C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.-M. Cultivar-Specific Changes in Primary and Secondary Metabolites in Pak Choi (Brassica Rapa, Chinensis Group) by Methyl Jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Juvik, J.A. Environmental Stress and Methyl Jasmonate-Mediated Changes in Flavonoid Concentrations and Antioxidant Activity in Broccoli Florets and Kale Leaf Tissues. HortScience 2013, 48, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Jones-Baumgardt, C.; Llewellyn, D.; Ying, Q.; Zheng, Y. Intensity of Sole-Source Light-Emitting Diodes Affects Growth, Yield, and Quality of Brassicaceae Microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Kavga, A.; Strati, I.F.; Sinanoglou, V.J.; Fotakis, C.; Sotiroudis, G.; Christodoulou, P.; Zoumpoulakis, P. Evaluating the Experimental Cultivation of Peppers in Low-Energy-Demand Greenhouses. An Interdisciplinary Study. J. Sci. Food Agric. 2019, 99, 781–789. [Google Scholar] [CrossRef]
- Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A. Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables. Molecules 2011, 16, 6816–6832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnham, M.W.; Wilson, P.E.; Stephenson, K.K.; Fahey, J.W. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed. 2004, 123, 60–65. [Google Scholar] [CrossRef]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of Climate and Genotype to Seasonal Variation in the Glucosinolate–Myrosinase System. I. Glucosinolate Content in Ten Cultivars of Brassica Oleracea Grown in Fall and Spring Seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar]
- Nuñez-Gómez, V.; Baenas, N.; Navarro-González, I.; García-Alonso, J.; Moreno, D.A.; González-Barrio, R.; Periago-Castón, M.J. Seasonal Variation of Health-Promoting Bioactives in Broccoli and Methyl-Jasmonate Pre-harvest Treatments to Enhance Their Contents. Foods 2020, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S.S.; Rao, K. Protease Inhibitors from Marine Actinobacteria as a Potential Source for Antimalarial Compound. PLoS ONE 2014, 9, e90972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-S.; Ku, K.-M.; Becker, T.M.; Juvik, J.A. Chemopreventive glucosinolate accumulation in various broccoli and collard tissues: Microfluidic-based targeted transcriptomics for by-product valorization. PLoS ONE 2017, 12, e0185112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, R.; Bisht, N.C. Biofortification of oilseed Brassica juncea with the anti-cancer compound glucoraphanin by suppressing GSL-ALK gene family. Sci. Rep. 2015, 5, 18005. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-P.; Wang, M.-L.; Chan, M.-H.; Chiu, Y.-S.; Chen, Y.-H. Antiobesity Activities of Indole-3-Carbinol in High-Fat-Diet–Induced Obese Mice. Nutrition 2011, 27, 463–470. [Google Scholar] [CrossRef]
- Michnovicz, J. Increased estrogen 2-hydroxylation in obese women using oral indole-3-carbinol. Int. J. Obes. 1998, 22, 227–229. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Marconett, C.N.; Sundar, S.; Tseng, M.N.; Tin, A.S.; Tran, K.Q.; Mahuron, K.M.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-Carbinol Downregulation of Telomerase Gene Expression Requires the Inhibition of Estrogen Receptor-Alpha and Sp1 Transcription Factor Interactions within the Htert Promoter and Mediates the G 1 Cell Cycle Arrest of Human Breast Cancer Cells. Carcinogenesis 2011, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enríquez, J.; Velázquez-Cruz, R.; Parra-Torres, A.; Gutiérrez-Sagal, R.; Larrea, F. The Anti-Estrogenic Activity of Indole-3-Carbinol in Neonatal Rat Osteoblasts Is Associated with the Estrogen Receptor Antagonist 2-Hydroxyestradiol. J. Endocrinol. Investig. 2016, 39, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Choi, J.-H.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Pre-harvest Methyl Jasmonate Treatment Enhances Cauliflower Chemoprotective Attributes Without a Loss in Postharvest Quality. Plant Foods Hum. Nutr. 2013, 68, 113–117. [Google Scholar] [CrossRef]
- Ku, K.-M.; Jeffery, E.; Juvik, J.A. Exogenous Methyl Jasmonate Treatment Increases Glucosinolate Biosynthesis and Quinone Reductase Activity in Kale Leaf Tissue. PLoS ONE 2014, 9, e103407. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.-M.; Becker, T.M.; Juvik, J.A. Transcriptome and Metabolome Analyses of Glucosinolates in Two Broccoli Cultivars Following Jasmonate Treatment for the Induction of Glucosinolate Defense to Trichoplusia ni (Hübner). Int. J. Mol. Sci. 2016, 17, 1135. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-C.; Matak, K.; Ku, K.-M. Methyl jasmonate treated broccoli: Impact on the production of glucosinolates and consumer preferences. Food Chem. 2019, 299, 125099. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; Benavente-García, A.G.; García-Viguera, C. Total and Individual Glucosinolate Contents in Inflorescences of Eight Broccoli Cultivars Grown under Various Climatic and Fertilisation Conditions. J. Sci. Food Agric. 2003, 83, 307–313. [Google Scholar] [CrossRef]
- Bohinc, T.; Trdan, S. Environmental Factors Affecting the Glucosinolate Content in Brassicaceae. J. Food Agric. Environ. 2012, 10, 357. [Google Scholar]
- Chae, S.-H.; Lee, O.N.; Park, H.Y.; Ku, K.-M. Seasonal Effects of Glucosinolate and Sugar Content Determine the Pungency of Small-Type (Altari) Radishes (Raphanus sativus L.). Plants 2022, 11, 312. [Google Scholar] [CrossRef]
- Clarke, J.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Pék, Z.; Daood, H.; Nagyné, M.; Neményi, A.; Helyes, L. Effect of environmental conditions and water status on the bioactive compounds of broccoli. Open Life Sci. 2013, 8, 777–787. [Google Scholar] [CrossRef]
- Mewis, I.; Khan, M.A.M.; Glawischnig, E.; Schreiner, M.; Ulrichs, C. Water Stress and Aphid Feeding Differentially Influence Metabolite Composition in Arabidopsis thaliana (L.). PLoS ONE 2012, 7, e48661. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.d.C.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [Green Version]
- Dini, M.; Raseira, M.D.C.B.; Scariotto, S.; Carra, B.; De Abreu, E.S.; Mello-Farias, P.; Cantillano, R.F.F. Color Shade Heritability of Peach Flesh. J. Agric. Sci. 2019, 11, 236. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Romero-Bravo, S.; Del Pozo, A. Productivity and Fruit Quality of Vaccinium Corymbosum Cv. Elliott under Photo-Selective Shading Nets. Sci. Hortic. 2013, 153, 143–149. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; Beauclair, P.; Bataillé, M.-P.; Avice, J.-C.; Trouverie, J.; Etienne, P.; Ourry, A. Light Restriction Delays Leaf Senescence in Winter Oilseed Rape (Brassica napus L.). J. Plant Growth Regul. 2013, 32, 506–518. [Google Scholar] [CrossRef]
- Francescangeli, N.; Martí, H.R.; Sangiacomo, M.A. Vegetative and Reproductive Plasticity of Broccoli at Three Levels of Incident Photosynthetically Active Radiation. Span. J. Agric. Res. 2007, 5, 389–401. [Google Scholar] [CrossRef] [Green Version]








| OF/AV | DTH | GDD (°C) | Solar Radiation (Wh/m2) | Precipitation (mm) |
|---|---|---|---|---|
| 2019 Fall | 79/74 | 902/878 | 3562 | 388 |
| 2020 Spring | 34/34 | 435/435 | 5553 | 199 |
| 2020 Fall | 74/74 | 646/646 | 3399 | 41 |
| Open-Field | Agrivoltaic | |
|---|---|---|
| Soil temperature | 20.2 ± 5.1 *** | 19.1 ± 4.1 |
| PPFD | 635 ± 59 *** | 369 ± 60 |
| Revenue | Amount |
|---|---|
| Benefits of annual cost reduction (USD) | 1896 |
| Annual cost reduction by AV (USD) | 1669 |
| co-benefits of carbon emissions reduction (USD) | 395 |
| Sum (USD) | 3960 |
| Revenue | Amount |
|---|---|
| Cultivation Area (a) | 3.24 |
| Profits per area (USD) | 116.9 |
| Annual broccoli profits under AV (USD) | 378.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, S.-H.; Kim, H.J.; Moon, H.-W.; Kim, Y.H.; Ku, K.-M. Agrivoltaic Systems Enhance Farmers’ Profits through Broccoli Visual Quality and Electricity Production without Dramatic Changes in Yield, Antioxidant Capacity, and Glucosinolates. Agronomy 2022, 12, 1415. https://doi.org/10.3390/agronomy12061415
Chae S-H, Kim HJ, Moon H-W, Kim YH, Ku K-M. Agrivoltaic Systems Enhance Farmers’ Profits through Broccoli Visual Quality and Electricity Production without Dramatic Changes in Yield, Antioxidant Capacity, and Glucosinolates. Agronomy. 2022; 12(6):1415. https://doi.org/10.3390/agronomy12061415
Chicago/Turabian StyleChae, Seung-Hun, Hye Joung Kim, Hyeon-Woo Moon, Yoon Hyung Kim, and Kang-Mo Ku. 2022. "Agrivoltaic Systems Enhance Farmers’ Profits through Broccoli Visual Quality and Electricity Production without Dramatic Changes in Yield, Antioxidant Capacity, and Glucosinolates" Agronomy 12, no. 6: 1415. https://doi.org/10.3390/agronomy12061415
APA StyleChae, S.-H., Kim, H. J., Moon, H.-W., Kim, Y. H., & Ku, K.-M. (2022). Agrivoltaic Systems Enhance Farmers’ Profits through Broccoli Visual Quality and Electricity Production without Dramatic Changes in Yield, Antioxidant Capacity, and Glucosinolates. Agronomy, 12(6), 1415. https://doi.org/10.3390/agronomy12061415