Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments
Abstract
:1. Introduction
2. Small Activating RNAs Are Involved in Locus-Specific Induction of Neural Genes
3. Small Activating RNAs Are Involved in Locus-Specific Induction of Cardiac Genes
4. Small Activating RNAs: New Insights into Cancer Therapy
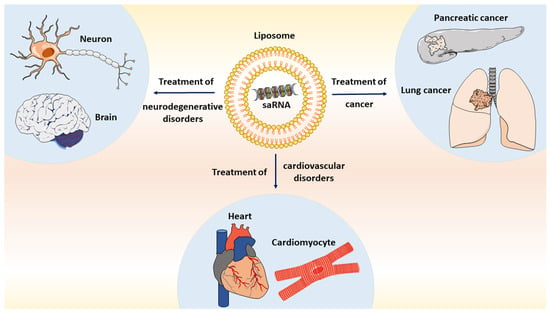
5. Towards the Development of New Therapeutic Agents
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aghamiri, S.; Mehrjardi, K.F.; Shabani, S.; Keshavarz-Fathi, M.; Kargar, S.; Rezaei, N. Nanoparticle-siRNA: A potential strategy for ovarian cancer therapy? Nanomedicine 2019, 14, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Bajan, S.; Hutvagner, G. RNA-Based Therapeutics: From Antisense Oligonucleotides to miRNAs. Cells 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed Sci. 2017, 13, 48–57. [Google Scholar] [PubMed]
- Sun, G.; Yeh, S.Y.; Yuan, C.W.; Chiu, M.J.; Yung, B.S.; Yen, Y. Molecular Properties, Functional Mechanisms, and Applications of Sliced siRNA. Mol. Ther. Nucleic Acids 2015, 4, e221. [Google Scholar] [CrossRef] [PubMed]
- Holoch, D.; Moazed, D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015, 16, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Volpe, T.; Martienssen, R.A. RNA interference and heterochromatin assembly. Cold Spring Harb. Perspect. Biol. 2011, 3, a003731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czech, B.; Hannon, G.J. Small RNA sorting: Matchmaking for Argonautes. Nat. Rev. Genet. 2011, 12, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Xiao, H.; Zhang, J.; Liang, X.J.; Huang, Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 2019, 37, 801–825. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M.; Kang, M.R.; Yang, G.; Li, L.-C. Targeted p21WAF1/CIP1 Activation by RNAa Inhibits Hepatocellular Carcinoma Cells. Nucleic Acid Ther. 2012, 22, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Jiang, Q.; Chang, N.; Wang, X.; Liu, C.; Xiong, J.; Cao, H.; Liang, Z. Small activating RNA binds to the genomic target site in a seed-region-dependent manner. Nucleic Acids Res. 2016, 44, 2274–2282. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Place, R.F.; Portnoy, V.; Huang, V.; Kang, M.R.; Kosaka, M.; Ho, M.K.C.; Li, L.C. Inducing gene expression by targeting promoter sequences using small activating RNAs. J. Biol. Methods 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Portnoy, V.; Huang, V.; Place, R.F.; Li, L.C. Small RNA and transcriptional upregulation. Wiley Interdiscip. Rev. RNA 2011, 2, 748–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modarresi, F.; Faghihi, M.A.; Lopez-Toledano, M.A.; Fatemi, R.P.; Magistri, M.; Brothers, S.P.; van der Brug, M.P.; Wahlestedt, C. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat. Biotechnol. 2012, 30, 453–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbarian, H.; Wagner, N.; Michiels, J.F.; Cuzin, F.; Wagner, K.D.; Rassoulzadegan, M. Small RNA-directed epigenetic programming of embryonic stem cell cardiac differentiation. Sci. Rep. 2017, 7, 41799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbarian, H.; Grandjean, V.; Cuzin, F.; Rassoulzadegan, M. A Network of Regulations by Small Non-Coding RNAs: The P-TEFb Kinase in Development and Pathology. Front. Genet. 2011, 2, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Zhang, L.; Luo, W.; Zhang, X. Characteristics of Antisense Transcript Promoters and the Regulation of Their Activity. Int. J. Mol. Sci. 2015, 17, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faghihi, M.; Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009, 10, 637–643. [Google Scholar] [CrossRef]
- Faghihi, M.A.; Kocerha, J.; Modarresi, F.; Engström, P.G.; Chalk, A.M.; Brothers, S.P.; Koesema, E.; St Laurent, G.; Wahlestedt, C. RNAi screen indicates widespread biological function for human natural antisense transcripts. PLoS ONE 2010, 5, e13177. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Saetrom, P.; Snøve, O.; Rossi, J.J. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16230–16235. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, T.; Hsieh, J.; Nakashima, K.; Taira, K.; Gage, F.H. A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 2004, 116, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Fimiani, C.; Goina, E.; Su, Q.; Gao, G.; Mallamaci, A. RNA activation of haploinsufficient Foxg1 gene in murine neocortex. Sci. Rep. 2016, 6, 39311. [Google Scholar] [CrossRef] [Green Version]
- Burns, T.C.; Verfaillie, C.M.; Low, W.C. Stem cells for ischemic brain injury: A critical review. J. Comp. Neurol. 2009, 515, 125–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diodato, A.; Pinzan, M.; Granzotto, M.; Mallamaci, A. Promotion of cortico-cerebral precursors expansion by artificial pri-miRNAs targeted against the Emx2 locus. Curr. Gene Ther. 2013, 13, 152–161. [Google Scholar] [CrossRef]
- Guerrini, R.; Parrini, E. Epilepsy in Rett syndrome, and CDKL5- and FOXG1-gene-related encephalopathies. Epilepsia 2012, 53, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.A.; Alcántara, S. BDNF/MAPK/ERK-induced BMP7 expression in the developing cerebral cortex induces premature radial glia differentiation and impairs neuronal migration. Cereb. Cortex 2010, 20, 2132–2144. [Google Scholar] [CrossRef] [Green Version]
- Antal, A.; Chaieb, L.; Moliadze, V.; Monte-Silva, K.; Poreisz, C.; Thirugnanasambandam, N.; Nitsche, M.A.; Shoukier, M.; Ludwig, H.; Paulus, W. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010, 3, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.V.; Escobar, M.L. A role for MAPK and PI-3K signaling pathways in brain-derived neurotrophic factor modification of conditioned taste aversion retention. Behav. Brain Res. 2011, 217, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.M.; Meynard, M.M.; Marshak, S.; Cohen-Cory, S. Synaptic maturation of the Xenopus retinotectal system: Effects of brain-derived neurotrophic factor on synapse ultrastructure. J. Comp. Neurol. 2010, 518, 972–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.C.; Maguschak, K.A.; Ye, K.; Jang, S.W.; Myers, K.M.; Ressler, K.J. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc. Natl. Acad. Sci. USA 2010, 107, 2675–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapleau, C.A.; Larimore, J.L.; Theibert, A.; Pozzo-Miller, L. Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: Fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J. Neurodev. Disord. 2009, 1, 185–196. [Google Scholar] [CrossRef] [Green Version]
- d’Ydewalle, C.; Sumner, C.J. Spinal Muscular Atrophy Therapeutics: Where do we Stand? Neurotherapeutics 2015, 12, 303–316. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, S.; Burlet, P.; Liu, Q.; Bertrandy, S.; Clermont, O.; Munnich, A.; Dreyfuss, G.; Melki, J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997, 16, 265–269. [Google Scholar] [CrossRef] [PubMed]
- d’Ydewalle, C.; Ramos, D.M.; Pyles, N.J.; Ng, S.Y.; Gorz, M.; Pilato, C.M.; Ling, K.; Kong, L.; Ward, A.J.; Rubin, L.L.; et al. The Antisense Transcript SMN-AS1 Regulates SMN Expression and Is a Novel Therapeutic Target for Spinal Muscular Atrophy. Neuron 2017, 93, 66–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turunen, M.P.; Husso, T.; Musthafa, H.; Laidinen, S.; Dragneva, G.; Laham-Karam, N.; Honkanen, S.; Paakinaho, A.; Laakkonen, J.P.; Gao, E.; et al. Epigenetic upregulation of endogenous VEGF-A reduces myocardial infarct size in mice. PLoS ONE 2014, 9, e89979. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, T.; Rao, K.; Yang, J.; Zhang, S.; Wang, S.; Liu, J.; Ye, Z. Up-regulation of VEGF by small activator RNA in human corpus cavernosum smooth muscle cells. J. Sex Med. 2011, 8, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Husso, T.; Ylä-Herttuala, S.; Turunen, M.P. A New Gene Therapy Approach for Cardiovascular Disease by Non-coding RNAs Acting in the Nucleus. Mol. Ther. Nucleic Acids 2014, 3, e197. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Taube, R.; Fujinaga, K.; Peterlin, B.M. P-TEFb containing cyclin K and Cdk9 can activate transcription via RNA. J. Biol. Chem. 2002, 277, 16873–16878. [Google Scholar] [CrossRef] [Green Version]
- Marshall, N.F.; Price, D.H. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J. Biol. Chem. 1995, 270, 12335–12338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, D.H. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell Biol. 2000, 20, 2629–2634. [Google Scholar] [CrossRef] [Green Version]
- Leucci, E.; De Falco, G.; Onnis, A.; Cerino, G.; Cocco, M.; Luzzi, A.; Crupi, D.; Tigli, C.; Bellan, C.; Tosi, P.; et al. The role of the Cdk9/Cyclin T1 complex in T cell differentiation. J. Cell Physiol. 2007, 212, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Pevarello, P.; Barbacid, M.; Bischoff, J.R. CDK inhibitors in cancer therapy: What is next? Trends Pharmacol. Sci. 2008, 29, 16–21. [Google Scholar] [CrossRef]
- Kaichi, S.; Takaya, T.; Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Ono, K.; Shimatsu, A.; Baba, S.; Heike, T.; Nakahata, T.; et al. Cyclin-dependent kinase 9 forms a complex with GATA4 and is involved in the differentiation of mouse ES cells into cardiomyocytes. J. Cell Physiol. 2011, 226, 248–254. [Google Scholar] [CrossRef]
- Tarhriz, V.; Wagner, K.D.; Masoumi, Z.; Molavi, O.; Hejazi, M.S.; Ghanbarian, H. CDK9 Regulates Apoptosis of Myoblast Cells by Modulation of microRNA-1 Expression. J. Cell Biochem. 2018, 119, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Wagner, N.; Ghanbarian, H.; Grandjean, V.; Gounon, P.; Cuzin, F.; Rassoulzadegan, M. RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse. Dev. Cell 2008, 14, 962–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Olson, E.N.; Bassel-Duby, R. Mending broken hearts: Cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013, 14, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Sayed, D.; Hong, C.; Chen, I.Y.; Lypowy, J.; Abdellatif, M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 2007, 100, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Peterlin, B.M.; Brogie, J.E.; Price, D.H. 7SK snRNA: A noncoding RNA that plays a major role in regulating eukaryotic transcription. Wiley Interdiscip. Rev. RNA 2012, 3, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Mattick, J.S. The State of Long Non-Coding RNA Biology. Noncoding RNA 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Mattick, J.S.; Rinn, J.L. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015, 22, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Burnett, J.C.; Rossi, J.J. The role of antisense long noncoding RNA in small RNA-triggered gene activation. RNA 2014, 20, 1916–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.C.; Okino, S.T.; Zhao, H.; Pookot, D.; Place, R.F.; Urakami, S.; Enokida, H.; Dahiya, R. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. USA 2006, 103, 17337–17342. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.; Sakurai, F.; Elbashir, S.; Foster, D.J.; Manoharan, M.; Corey, D.R. Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter. Chem. Biol. 2010, 17, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Werner, A. Biological functions of natural antisense transcripts. BMC Biol. 2013, 11, 31. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Place, R.F.; Jia, Z.J.; Pookot, D.; Dahiya, R.; Li, L.C. Antitumor effect of dsRNA-induced p21(WAF1/CIP1) gene activation in human bladder cancer cells. Mol. Cancer Ther. 2008, 7, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Place, R.F.; Wang, J.; Noonan, E.J.; Meyers, R.; Manoharan, M.; Charisse, K.; Duncan, R.; Huang, V.; Wang, X.; Li, L.C. Formulation of Small Activating RNA Into Lipidoid Nanoparticles Inhibits Xenograft Prostate Tumor Growth by Inducing p21 Expression. Mol. Ther. Nucleic Acids 2012, 1, e15. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Long, M.; Han, Y.; Wang, X.; Lin, F.; Ren, J.; He, T.; Zhang, H. p21WAF1/CIP1 gene transcriptional activation exerts cell growth inhibition and enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC Cancer 2010, 10, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.M.; Dai, C.; Huang, Y.; Zheng, C.F.; Dong, Q.Z.; Wang, G.; Li, X.W.; Zhang, X.F.; Li, B.; Chen, G. Anti-cancer effects of p21WAF1/CIP1 transcriptional activation induced by dsRNAs in human hepatocellular carcinoma cell lines. Acta Pharmacol. Sin. 2011, 32, 939–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frixen, U.H.; Behrens, J.; Sachs, M.; Eberle, G.; Voss, B.; Warda, A.; Löchner, D.; Birchmeier, W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 1991, 113, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Richards, F.M.; McKee, S.A.; Rajpar, M.H.; Cole, T.R.; Evans, D.G.; Jankowski, J.A.; McKeown, C.; Sanders, D.S.; Maher, E.R. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum. Mol. Genet. 1999, 8, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Zandsalimi, F.; Talaei, S.; Noormohammad Ahari, M.; Aghamiri, S.; Raee, P.; Roshanzamiri, S.; Yarian, F.; Bandehpour, M.; Zohrab Zadeh, Z. Antimicrobial peptides: A promising strategy for lung cancer drug discovery? Expert Opin. Drug Discov. 2020, 15, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Plummer, R.; Meyer, T.; Sodergren, M.H.; Basu, B.; Chee, C.E.; Huang, K.W.; Palmer, D.H.; Ma, Y.T.; Evans, T.R.J.; et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-α, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 2020, 26, 3936–3946. [Google Scholar] [CrossRef]
- Kramer, E.D.; Abrams, S.I. Granulocytic Myeloid-Derived Suppressor Cells as Negative Regulators of Anticancer Immunity. Front. Immunol. 2020, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Liu, X.; Wang, J.; Li, F.; Li, C.; Shan, B. Up-regulation of p21WAF1/CIP1 by small activating RNA inhibits the in vitro and in vivo growth of pancreatic cancer cells. Tumori 2012, 98, 804–811. [Google Scholar] [CrossRef]
- Yang, K.; Zheng, X.Y.; Qin, J.; Wang, Y.B.; Bai, Y.; Mao, Q.Q.; Wan, Q.; Wu, Z.M.; Xie, L.P. Up-regulation of p21WAF1/Cip1 by saRNA induces G1-phase arrest and apoptosis in T24 human bladder cancer cells. Cancer Lett. 2008, 265, 206–214. [Google Scholar] [CrossRef]
- Qin, Q.; Lin, Y.W.; Zheng, X.Y.; Chen, H.; Mao, Q.Q.; Yang, K.; Huang, S.J.; Zhao, Z.Y. RNAa-mediated overexpression of WT1 induces apoptosis in HepG2 cells. World J. Surg. Oncol. 2012, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.; Zheng, X.; Yang, K.; Qin, J.; Bai, Y.; Jia, X.; Li, Y.; Xie, L. Suppression of migration and invasion of PC3 prostate cancer cell line via activating E-cadherin expression by small activating RNA. Cancer Investig. 2010, 28, 1013–1018. [Google Scholar] [CrossRef]
- Mao, Q.; Li, Y.; Zheng, X.; Yang, K.; Shen, H.; Qin, J.; Bai, Y.; Kong, D.; Jia, X.; Xie, L. Up-regulation of E-cadherin by small activating RNA inhibits cell invasion and migration in 5637 human bladder cancer cells. Biochem. Biophys. Res. Commun. 2008, 375, 566–570. [Google Scholar] [CrossRef]
- Junxia, W.; Ping, G.; Yuan, H.; Lijun, Z.; Jihong, R.; Fang, L.; Min, L.; Xi, W.; Ting, H.; Ke, D.; et al. Double strand RNA-guided endogeneous E-cadherin up-regulation induces the apoptosis and inhibits proliferation of breast carcinoma cells in vitro and in vivo. Cancer Sci. 2010, 101, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Place, R.F.; Huang, V.; Wang, X.; Noonan, E.J.; Magyar, C.E.; Huang, J.; Li, L.C. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 2010, 70, 10182–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Meng, L.; Xu, F.; Lian, J.; Xu, Y.; Xie, X.; Wang, X.; He, H.; Wang, C.; Zhu, Y. Enhanced wild-type p53 expression by small activating RNA dsP53-285 induces cell cycle arrest and apoptosis in pheochromocytoma cell line PC12. Oncol. Rep. 2017, 38, 3160–3166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Pan, S.; Gu, Y.; Guo, S.; Dai, Q.; Yu, Y.; Zhang, W. Small activating RNA restores the activity of the tumor suppressor HIC-1 on breast cancer. PLoS ONE 2014, 9, e86486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Shen, J.; Xie, Y.Q.; Lin, Y.W.; Qin, J.; Mao, Q.Q.; Zheng, X.Y.; Xie, L.P. Promoter-targeted double-stranded small RNAs activate PAWR gene expression in human cancer cells. Int. J. Biochem. Cell Biol. 2013, 45, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Kang, M.R.; Wang, J.; Huang, V.; Place, R.F.; Sun, Y.; Li, L.C. Targeted induction of endogenous NKX3-1 by small activating RNA inhibits prostate tumor growth. Prostate 2013, 73, 1591–1601. [Google Scholar] [CrossRef]
- Zeng, T.; Duan, X.; Zhu, W.; Liu, Y.; Wu, W.; Zeng, G. SaRNA-mediated activation of TRPV5 reduces renal calcium oxalate deposition in rat via decreasing urinary calcium excretion. Urolithiasis 2018, 46, 271–278. [Google Scholar] [CrossRef]
- Kang, M.R.; Park, K.H.; Lee, C.W.; Lee, M.Y.; Han, S.B.; Li, L.C.; Kang, J.S. Small activating RNA induced expression of VHL gene in renal cell carcinoma. Int. J. Biochem. Cell Biol. 2018, 97, 36–42. [Google Scholar] [CrossRef]
- Xia, W.; Li, D.; Wang, G.; Ni, J.; Zhuang, J.; Ha, M.; Wang, J.; Ye, Y. Small activating RNA upregulates NIS expression: Promising potential for hepatocellular carcinoma endoradiotherapy. Cancer Gene Ther. 2016, 23, 333–340. [Google Scholar] [CrossRef]
- Kang, M.R.; Yang, G.; Place, R.F.; Charisse, K.; Epstein-Barash, H.; Manoharan, M.; Li, L.C. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Res. 2012, 72, 5069–5079. [Google Scholar] [CrossRef] [Green Version]
- Reebye, V.; Sætrom, P.; Mintz, P.J.; Huang, K.W.; Swiderski, P.; Peng, L.; Liu, C.; Liu, X.; Lindkaer-Jensen, S.; Zacharoulis, D.; et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology 2014, 59, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, H.; Wen, X.; Chen, X.; Wu, L.; Liu, W.; Habib, N.A.; Bie, P.; Xia, F. C/EBPα Short-Activating RNA Suppresses Metastasis of Hepatocellular Carcinoma through Inhibiting EGFR/β-Catenin Signaling Mediated EMT. PLoS ONE 2016, 11, e0153117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Feng, C.L.; Zheng, W.S.; Huang, S.; Zhang, W.X.; Wu, H.N.; Zhan, Y.; Han, Y.X.; Wu, S.; Jiang, J.D. Tumor-selective lipopolyplex encapsulated small active RNA hampers colorectal cancer growth in vitro and in orthotopic murine. Biomaterials 2017, 141, 13–28. [Google Scholar] [CrossRef]
- Yoon, S.; Huang, K.W.; Reebye, V.; Mintz, P.; Tien, Y.W.; Lai, H.S.; Sætrom, P.; Reccia, I.; Swiderski, P.; Armstrong, B.; et al. Targeted Delivery of C/EBPα -saRNA by Pancreatic Ductal Adenocarcinoma-specific RNA Aptamers Inhibits Tumor Growth In Vivo. Mol. Ther. 2016, 24, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jiang, W.; Hu, Q.; Li, L.C.; Dong, L.; Chen, R.; Zhang, Y.; Tang, Y.; Thrasher, J.B.; Liu, C.B.; et al. Enhancing DPYSL3 gene expression via a promoter-targeted small activating RNA approach suppresses cancer cell motility and metastasis. Oncotarget 2016, 7, 22893–22910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Li, M.; Yuan, H.; Zhan, Y.; Xu, H.; Wang, S.; Yang, W.; Liu, J.; Ye, Z.; Li, L.C. saRNA guided iNOS up-regulation improves erectile function of diabetic rats. J. Urol. 2013, 190, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jiang, K.; Jiang, P.; He, H.; Chen, K.; Shao, J.; Deng, G. Mechanism of Notch1-saRNA-1480 reversing androgen sensitivity in human metastatic castration-resistant prostate cancer. Int. J. Mol. Med. 2020, 46, 265–279. [Google Scholar] [CrossRef]
- Huang, K.W.; Reebye, V.; Czysz, K.; Ciriello, S.; Dorman, S.; Reccia, I.; Lai, H.S.; Peng, L.; Kostomitsopoulos, N.; Nicholls, J.; et al. Liver Activation of Hepatocellular Nuclear Factor-4α by Small Activating RNA Rescues Dyslipidemia and Improves Metabolic Profile. Mol. Ther. Nucleic Acids 2020, 19, 361–370. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, X.; Huang, Y.; Tang, M.; Wu, L. Upregulation of FHIT gene expression in endometrial carcinoma by RNA activation. Int. J. Clin. Exp. Pathol. 2020, 13, 1372–1380. [Google Scholar] [PubMed]
- Wahlestedt, C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat. Rev. Drug Discov. 2013, 12, 433–446. [Google Scholar] [CrossRef]
- Poller, W.; Tank, J.; Skurk, C.; Gast, M. Cardiovascular RNA interference therapy: The broadening tool and target spectrum. Circ. Res. 2013, 113, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Hedman, M.; Hartikainen, J.; Ylä-Herttuala, S. Progress and prospects: Hurdles to cardiovascular gene therapy clinical trials. Gene Ther. 2011, 18, 743. [Google Scholar] [CrossRef]
- Cotrim, A.P.; Baum, B.J. Gene therapy: Some history, applications, problems, and prospects. Toxicol. Pathol. 2008, 36, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J. The challenge of gene therapy and DNA delivery. J. Pharm. Pharmacol. 2001, 53, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Jafarlou, M.; Baradaran, B.; Saedi, T.; Jafarlou, V.; Shanehbandi, D.; Maralani, M.; Othman, F. An overview of the history, applications, advantages, disadvantages and prospects of gene therapy. J. Biol. Regul. Homeost Agents 2016, 30, 315–321. [Google Scholar] [PubMed]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Patel, A.K.; Kauffman, K.J.; Fenton, O.S.; Webber, M.J.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Polymer-Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew. Chem. Int. Ed. Engl. 2016, 55, 13808–13812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ickenstein, L.M.; Garidel, P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef]
- Watts, J.K.; Yu, D.; Charisse, K.; Montaillier, C.; Potier, P.; Manoharan, M.; Corey, D.R. Effect of chemical modifications on modulation of gene expression by duplex antigene RNAs that are complementary to non-coding transcripts at gene promoters. Nucleic Acids Res. 2010, 38, 5242–5259. [Google Scholar] [CrossRef] [Green Version]
- Wahlestedt, C.; Salmi, P.; Good, L.; Kela, J.; Johnsson, T.; Hökfelt, T.; Broberger, C.; Porreca, F.; Lai, J.; Ren, K.; et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl. Acad. Sci. USA 2000, 97, 5633–5638. [Google Scholar] [CrossRef] [Green Version]
- Veedu, R.N.; Wengel, J. Locked nucleic acid as a novel class of therapeutic agents. RNA Biol. 2009, 6, 321–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Qu, Z.; Kim, S.; Shi, V.; Liao, B.; Kraft, P.; Bandaru, R.; Wu, Y.; Greenberger, L.; Horak, I. Down-modulation of cancer targets using locked nucleic acid (LNA)-based antisense oligonucleotides without transfection. Gene Ther. 2011, 18, 326. [Google Scholar] [CrossRef]
- Mook, O.; Vreijling, J.; Wengel, S.L.; Wengel, J.; Zhou, C.; Chattopadhyaya, J.; Baas, F.; Fluiter, K. In vivo efficacy and off-target effects of locked nucleic acid (LNA) and unlocked nucleic acid (UNA) modified siRNA and small internally segmented interfering RNA (sisiRNA) in mice bearing human tumor xenografts. Artif. DNA PNA XNA 2010, 1, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Fluiter, K.; ten Asbroek, A.L.; de Wissel, M.B.; Jakobs, M.E.; Wissenbach, M.; Olsson, H.; Olsen, O.; Oerum, H.; Baas, F. In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res. 2003, 31, 953–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirin, M.; Winkler, J. Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert Opin. Biol. Ther. 2013, 13, 875–888. [Google Scholar] [CrossRef]
- Bennett, C.F.; Swayze, E.E. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef]
- Shim, M.S.; Kwon, Y.J. Efficient and targeted delivery of siRNA in vivo. FEBS J. 2010, 277, 4814–4827. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annu. Rev. Biomed. Eng. 2011, 13, 507–530. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.; Carver, K.; Cao, C.; Ming, X. Receptors, endocytosis, and trafficking: The biological basis of targeted delivery of antisense and siRNA oligonucleotides. J. Drug Target. 2013, 21, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Aghamiri, S.; Talaei, S.; Roshanzamiri, S.; Zandsalimi, F.; Fazeli, E.; Aliyu, M.; Kheiry Avarvand, O.; Ebrahimi, Z.; Keshavarz-Fathi, M.; Ghanbarian, H. Delivery of genome editing tools: A promising strategy for HPV-related cervical malignancy therapy. Expert Opin. Drug Deliv. 2020, 17, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Yee, F.; Ericson, H.; Reis, D.J.; Wahlestedt, C. Cellular uptake of intracerebroventricularly administered biotin- or digoxigenin-labeled antisense oligodeoxynucleotides in the rat. Cell Mol. Neurobiol. 1994, 14, 475–486. [Google Scholar] [CrossRef]
- Rigo, F.; Hua, Y.; Krainer, A.R.; Bennett, C.F. Antisense-based therapy for the treatment of spinal muscular atrophy. J. Cell Biol. 2012, 199, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. Immunogenic BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]

| Advantages | Disadvantages |
|---|---|
| Effective gene activation | Poor cellular uptake |
| Locus-specific activation of gene transcription, including undruggable targets | High sensitivity to RNase degradation |
| Easy to manufacture | Renal clearance |
| Cost-effectiveness | Repeated administration |
| Low toxicity | Off-target effects |
| Easy large-scale production | Activation of Toll-like receptors |
| Poor immunogenicity |
| Disease Condition | Gene | Comments | Ref. |
|---|---|---|---|
| Advanced liver cancer | CEBPA | The first clinical trial for saRNA-based therapeutics (NCT ID: NCT02716012; company: Mina Alpha Limited; phase 1). MTL-CEBPA shows favorable safety and promising synergistic effects in combination with TKIs. | [64] |
| Adult solid tumors | CEBPA | A new clinical trial of MTL-CEBPA in combination with pembrolizumab (NCT ID: NCT04105335; Phase 1; recruitment status: Recruiting). | [65] |
| Prostate cancer | P21 | Proliferation inhibition and tumor shrinkage. | [58] |
| Hepatocellular carcinoma (HCC) | P21 | Cell cycle arrest and inhibition of invasion and migration. | [10] |
| Non-small-cell lung carcinomas | P21 | In vitro: Proliferation inhibition, cell cycle arrest, and apoptosis induction. In vivo: Inhibition of tumor growth. | [59] |
| Pancreatic cancer | P21 | In vitro: Proliferation inhibition, cell cycle arrest, and apoptosis induction. In vivo: Inhibition of tumor growth; high safety. | [66] |
| Bladder cancer | P21 | Proliferation inhibition, cell cycle arrest, and apoptosis induction. | [67] |
| HCC | WT1 | Proliferation inhibition and apoptosis induction. | [68] |
| Prostate cancer | Ecad | Inhibition of invasion and migration. | [69] |
| Bladder cancer | Ecad | Inhibition of invasion and migration. | [70] |
| Breast cancer | Ecad | In vitro: Proliferation inhibition, cell cycle arrest, apoptosis induction, and inhibition of invasion and migration. In vivo: Tumor growth inhibition | [71] |
| Prostate cancer | KLF4 | Proliferation inhibition, cell cycle arrest, apoptosis induction, and inhibition of invasion and migration. | [72] |
| Malignant pheochromocytoma | TP53 | In vitro: Cell cycle arrest, proliferation inhibition, and apoptosis induction. In vivo: Tumor shrinkage. | [73] |
| Breast cancer | HIC-1 | Proliferation inhibition and apoptosis induction. | [74] |
| Bladder and prostate cancer | PAWR | Proliferation inhibition and apoptosis induction. | [75] |
| Prostate cancer | NKX3-1 | In vitro: Proliferation inhibition, cell cycle arrest, apoptosis induction. In vivo: Tumor growth inhibition. | [76] |
| Nephrolithiasis | TRPV5 | In vitro: TRPV5 expression induction. In vivo: TRPV5 expression induction and reduction in the formation of CaOx kidney stone. | [77] |
| Renal cell carcinoma | VHL | Cell growth inhibition and apoptosis induction. | [78] |
| HCC | NIS | Apoptosis induction and viability reduction of cancer cells. | [79] |
| Bladder cancer | P21 | Tumor Shrinkage | [80] |
| HCC | CEBPA | In vitro: CEBPA overexpression. In vivo: Tumor growth inhibition and tumor shrinkage. | [64] |
| HCC | CEBPA | In vitro: Proliferation inhibition. In vivo: Tumor burden reduction. | [81] |
| HCC | CEBPA | In vitro: Cell migration and invasion inhibition. In vivo: Metastasis inhibition | [82] |
| Colorectal cancer | P21 | In vitro: Apoptosis induction, proliferation inhibition, and cell migration and invasion inhibition. In vivo: Tumor growth inhibition. | [83] |
| Pancreatic ductal adenocarcinoma | CEBPA | In vitro: Proliferation inhibition. In vivo: Tumor shrinkage. | [84] |
| Prostate cancer | DPYSL3 | In vitro: Proliferation inhibition and cell migration and invasion inhibition. In vivo: Metastasis inhibition | [85] |
| Diabetes-induced erectile dysfunction | Nos2 | In vitro: iNos overexpression. In vivo: iNos overexpression and enhancement of peak intracavernous pressure. | [86] |
| Human metastatic castration-resistant prostate cancer | Notch1 | In vitro: Cell migration and invasion suppression, cell cycle arrest, and apoptosis inhibition. In vivo: Tumor growth inhibition and suppression of VEGF and AR pathways mechanisms. | [87] |
| Non-alcoholic fatty liver disease | HNF4A | In vitro: Increase in the expression level of HNF4A, CYP450, CYP3A4, CYP3A5, and CYP3A7. In vivo: Liver triglyceride reduction, high-density lipoprotein/low-density lipoprotein (HDL/LDL) ratio enhancement, and white adipose tissue/body weight ratio reduction. | [88] |
| Endometrial carcinoma | FHIT | Proliferation, invasion, and metastasis inhibition. | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanbarian, H.; Aghamiri, S.; Eftekhary, M.; Wagner, N.; Wagner, K.-D. Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments. Cells 2021, 10, 591. https://doi.org/10.3390/cells10030591
Ghanbarian H, Aghamiri S, Eftekhary M, Wagner N, Wagner K-D. Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments. Cells. 2021; 10(3):591. https://doi.org/10.3390/cells10030591
Chicago/Turabian StyleGhanbarian, Hossein, Shahin Aghamiri, Mohamad Eftekhary, Nicole Wagner, and Kay-Dietrich Wagner. 2021. "Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments" Cells 10, no. 3: 591. https://doi.org/10.3390/cells10030591
APA StyleGhanbarian, H., Aghamiri, S., Eftekhary, M., Wagner, N., & Wagner, K.-D. (2021). Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments. Cells, 10(3), 591. https://doi.org/10.3390/cells10030591