Cellular Therapy via Spermatogonial Stem Cells for Treating Impaired Spermatogenesis, Non-Obstructive Azoospermia
Abstract
:1. Male Infertility
1.1. Azoospermia
1.1.1. Pretesticular Causes
1.1.2. Testicular Causes
1.1.3. Post-Testicular Causes
1.1.4. Other Causes of Male Infertility
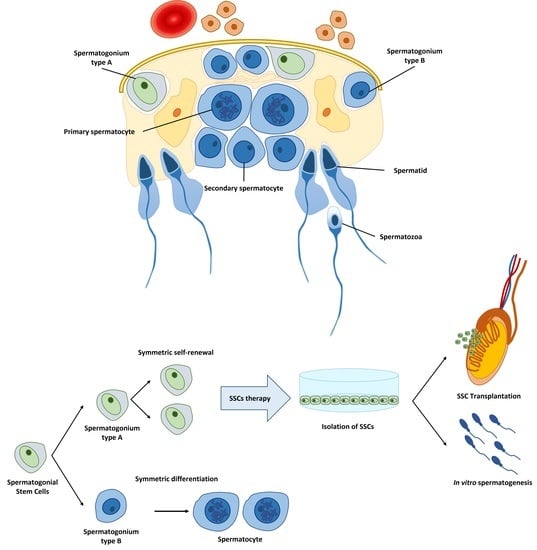
2. Spermatogonial Stem Cell as New Option to Treat Impaired Spermatogenesis
2.1. Spermatogonial Stem Cell Transplantation for Regeneration
2.1.1. Testicular Biopsy/Tissue
2.1.2. SSCs Isolation
2.1.3. SSCs Culturing and Expansion
2.1.4. SSCs Transplantation
2.2. In Vitro Spermatogenesis Using Spermatogonial Stem Cells
3. Other Stem Cell Types (iPSCs, ESCs, VSELs, and MSCs)
4. Conclusive Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hull, M.G.; Glazener, C.M.; Kelly, N.J.; Conway, D.I.; Foster, P.A.; Hinton, R.A.; Coulson, C.; Lambert, P.A.; Watt, E.M.; Desai, K.M. Population study of causes, treatment, and outcome of infertility. Br. Med. J. (Clin. Res. Ed.) 1985, 291, 1693–1697. [Google Scholar] [CrossRef] [Green Version]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmoghadam, Z.; Aghebati-Maleki, L.; Motalebnezhad, M.; Yousefi, B.; Yousefi, M. Current approaches for the treatment of male infertility with stem cell therapy. J. Cell Physiol. 2018, 233, 6455–6469. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Sun, H.; Gong, T.T.; Jiang, Y.T.; Zhang, S.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef]
- Bak, C.W.; Seok, H.H.; Song, S.H.; Kim, E.S.; Her, Y.S.; Yoon, T.K. Hormonal imbalances and psychological scars left behind in infertile men. J. Androl. 2012, 33, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Slade, P.; O’Neill, C.; Simpson, A.J.; Lashen, H. The relationship between perceived stigma, disclosure patterns, support and distress in new attendees at an infertility clinic. Hum. Reprod. 2007, 22, 2309–2317. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.K.; Elliott, P.; Katz, P.P.; Smith, J.F. Time costs of fertility care: The hidden hardship of building a family. Fertil. Steril. 2013, 99, 2025–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.E.; Farr, S.L.; Jamieson, D.J.; Warner, L.; Macaluso, M. Infertility services reported by men in the United States: National survey data. Fertil. Steril. 2009, 91, 2466–2470. [Google Scholar] [CrossRef]
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.M.; Spira, A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swan, S.H.; Elkin, E.P.; Fenster, L. The question of declining sperm density revisited: An analysis of 101 studies published 1934-1996. Environ. Health Perspect. 2000, 108, 961–966. [Google Scholar] [CrossRef]
- Mishra, P.; Negi, M.P.S.; Srivastava, M.; Singh, K.; Rajender, S. Decline in seminal quality in Indian men over the last 37 years. Reprod. Biol. Endocrinol. 2018, 16, 103. [Google Scholar] [CrossRef] [Green Version]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zhankina, R.; Baghban, N.; Askarov, M.; Saipiyeva, D.; Ibragimov, A.; Kadirova, B.; Khoradmehr, A.; Nabipour, I.; Shirazi, R.; Zhanbyrbekuly, U.; et al. Mesenchymal stromal/stem cells and their exosomes for restoration of spermatogenesis in non-obstructive azoospermia: A systemic review. Stem Cell Res. Ther. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstrom, W.J.G.; Palmert, M.R.; Corona, G.; Dohle, G.R.; et al. Paediatric and adult-onset male hypogonadism. Nat. Rev. Dis. Primers 2019, 5, 38. [Google Scholar] [CrossRef]
- Damsgaard, J.; Joensen, U.N.; Carlsen, E.; Erenpreiss, J.; Blomberg Jensen, M.; Matulevicius, V.; Zilaitiene, B.; Olesen, I.A.; Perheentupa, A.; Punab, M.; et al. Varicocele Is Associated with Impaired Semen Quality and Reproductive Hormone Levels: A Study of 7035 Healthy Young Men from Six European Countries. Eur. Urol. 2016, 70, 1019–1029. [Google Scholar] [CrossRef]
- Finelli, R.; Leisegang, K.; Finocchi, F.; De Masi, S.; Agarwal, A.; Damiani, G. The impact of autoimmune systemic inflammation and associated medications on male reproductive health in patients with chronic rheumatological, dermatological, and gastroenterological diseases: A systematic review. Am. J. Reprod. Immunol. 2021, 85, e13389. [Google Scholar] [CrossRef]
- Chehab, M.; Madala, A.; Trussell, J.C. On-label and off-label drugs used in the treatment of male infertility. Fertil. Steril. 2015, 103, 595–604. [Google Scholar] [CrossRef]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Louis, G.M. The relationship between male BMI and waist circumference on semen quality: Data from the LIFE study. Hum. Reprod. 2015, 30, 493–494. [Google Scholar] [CrossRef] [Green Version]
- Taha, E.A.; Ez-Aldin, A.M.; Sayed, S.K.; Ghandour, N.M.; Mostafa, T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology 2012, 80, 822–825. [Google Scholar] [CrossRef]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, A.J.; Afeiche, M.C.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Tanrikut, C.; Petrozza, J.C.; Chavarro, J.E. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Hum. Reprod. 2014, 29, 2575–2582. [Google Scholar] [CrossRef] [Green Version]
- Nargund, V.H. Effects of psychological stress on male fertility. Nat. Rev. Urol. 2015, 12, 373–382. [Google Scholar] [CrossRef]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab. J. Urol. 2018, 16, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Jarow, J.P.; Espeland, M.A.; Lipshultz, L.I. Evaluation of the azoospermic patient. J. Urol. 1989, 142, 62–65. [Google Scholar] [CrossRef]
- Sigman, M.; Jarow, J.P. Endocrine evaluation of infertile men. Urology 1997, 50, 659–664. [Google Scholar] [CrossRef]
- Urology, P.C.o.t.A.S.f.R.M.i.c.w.t.S.f.M.R.a. The management of infertility due to obstructive azoospermia. Fertil. Steril. 2008, 90, S121–S124. [Google Scholar] [CrossRef]
- Liu, P.Y.; Handelsman, D.J. The present and future state of hormonal treatment for male infertility. Hum. Reprod. Update 2003, 9, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Sussman, E.M.; Chudnovsky, A.; Niederberger, C.S. Hormonal evaluation of the infertile male: Has it evolved? Urol. Clin North. Am. 2008, 35, 147–155. [Google Scholar] [CrossRef]
- Maya-Nuñez, G.; Zenteno, J.C.; Ulloa-Aguirre, A.; Kofman-Alfaro, S.; Mendez, J.P. A recurrent missense mutation in the KAL gene in patients with X-linked Kallmann’s syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 1650–1653. [Google Scholar] [CrossRef]
- Bhasin, S.; Ma, K.; Sinha, I.; Limbo, M.; Taylor, W.E.; Salehian, B. The genetic basis of male infertility. Endocrinol. Metab. Clin. North. Am. 1998, 27, 783–805. [Google Scholar] [CrossRef]
- Darling, D.S.; Belker, A.M. Re: The genetics of male infertility. J. Urol. 1997, 158, 550–551. [Google Scholar] [CrossRef]
- Aiman, J.; Griffin, J.E.; Gazak, J.M.; Wilson, J.D.; MacDonald, P.C. Androgen insensitivity as a cause of infertility in otherwise normal men. N. Engl. J. Med. 1979, 300, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Schlegel, P.N. Endocrine manipulation in male infertility. Urol. Clin North. Am. 2008, 35, 303–318. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.I.; O’Donnell, L.; Meachem, S.J.; Stanton, P.G.; de, K.; Pratis, K.; Robertson, D.M. Hormonal regulation of spermatogenesis in primates and man: Insights for development of the male hormonal contraceptive. J. Androl. 2002, 23, 149–162. [Google Scholar]
- Eggert-Kruse, W.; Schwalbach, B.; Gerhard, I.; Tilgen, W.; Runnebaum, B. Influence of serum prolactin on semen characteristics and sperm function. Int. J. Fertil. 1991, 36, 243–251. [Google Scholar] [PubMed]
- Burrows, P.J.; Schrepferman, C.G.; Lipshultz, L.I. Comprehensive office evaluation in the new millennium. Urol. Clin North. Am. 2002, 29, 873–894. [Google Scholar] [CrossRef]
- Siddiq, F.M.; Sigman, M. A new look at the medical management of infertility. Urol. Clin North. Am. 2002, 29, 949–963. [Google Scholar] [CrossRef]
- Jane, J.A., Jr.; Laws, E.R., Jr. The surgical management of pituitary adenomas in a series of 3,093 patients. J. Am. Coll. Surg. 2001, 193, 651–659. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 2015, 30, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadziselimovic, F.; Hadziselimovic, N.O.; Demougin, P.; Oakeley, E.J. Testicular gene expression in cryptorchid boys at risk of azoospermia. Sex. Dev. 2011, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Richiardi, L.; Nordenskjold, A.; Kaijser, M.; Akre, O. Age at surgery for undescended testis and risk of testicular cancer. N. Engl. J. Med. 2007, 356. [Google Scholar] [CrossRef] [Green Version]
- Visser, A.J.; Heyns, C.F. Testicular function after torsion of the spermatic cord. BJU Int. 2003, 92, 200–203. [Google Scholar] [CrossRef]
- DaJusta, D.G.; Granberg, C.F.; Villanueva, C.; Baker, L.A. Contemporary review of testicular torsion: New concepts, emerging technologies and potential therapeutics. J. Pediatr. Urol. 2013, 9, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansbach, J.M.; Forbes, P.; Peters, C. Testicular torsion and risk factors for orchiectomy. Arch. Pediatr. Adolesc Med. 2005, 159, 1167–1171. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef]
- Werner, C.A. Mumps orchitis and testicular atrophy; a factor in male sterility. Ann. Intern. Med. 1950, 32, 1075–1086. [Google Scholar] [CrossRef]
- Cocuzza, M.; Alvarenga, C.; Pagani, R. The epidemiology and etiology of azoospermia. Clinics 2013, 68 (Suppl. 1), 15–26. [Google Scholar] [CrossRef]
- Ferlin, A.; Raicu, F.; Gatta, V.; Zuccarello, D.; Palka, G.; Foresta, C. Male infertility: Role of genetic background. Reprod. Biomed. Online 2007, 14, 734–745. [Google Scholar] [CrossRef]
- Bahadur, G.; Ralph, D. Gonadal tissue cryopreservation in boys with paediatric cancers. Hum. Reprod. 1999, 14, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Gandini, L.; Sgrò, P.; Lombardo, F.; Paoli, D.; Culasso, F.; Toselli, L.; Tsamatropoulos, P.; Lenzi, A. Effect of chemo- or radiotherapy on sperm parameters of testicular cancer patients. Hum. Reprod. 2006, 21, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, T.F. The effects of x irradiation on male human fertility. Br. J. Radiol 1966, 39, 901–907. [Google Scholar] [CrossRef]
- Jacob, A.; Barker, H.; Goodman, A.; Holmes, J. Recovery of spermatogenesis following bone marrow transplantation. Bone Marrow Transpl. 1998, 22, 277–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, J.E.; Hawley, J.; Levy, W.; Gooley, T.; Buckner, C.D.; Deeg, H.J.; Doney, K.; Storb, R.; Sullivan, K.; Witherspoon, R.; et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 1996, 87, 3045–3052. [Google Scholar] [CrossRef]
- Gbinigie, K.; Frie, K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open 2020, 4. [Google Scholar] [CrossRef]
- Kinsella, T.J.; Trivette, G.; Rowland, J.; Sorace, R.; Miller, R.; Fraass, B.; Steinberg, S.M.; Glatstein, E.; Sherins, R.J. Long-term follow-up of testicular function following radiation therapy for early-stage Hodgkin’s disease. J. Clin. Oncol. 1989, 7, 718–724. [Google Scholar] [CrossRef]
- Meistrich, M.L.; Wilson, G.; Brown, B.W.; da Cunha, M.F.; Lipshultz, L.I. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer 1992, 70, 2703–2712. [Google Scholar] [CrossRef] [Green Version]
- Meistrich, M.L.; Wilson, G.; Mathur, K.; Fuller, L.M.; Rodriguez, M.A.; McLaughlin, P.; Romaguera, J.E.; Cabanillas, F.F.; Ha, C.S.; Lipshultz, L.I.; et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine, and prednisone chemotherapy for Hodgkin’s disease. J. Clin. Oncol. 1997, 15, 3488–3495. [Google Scholar] [CrossRef]
- Byrne, J.; Mulvihill, J.J.; Myers, M.H.; Connelly, R.R.; Naughton, M.D.; Krauss, M.R.; Steinhorn, S.C.; Hassinger, D.D.; Austin, D.F.; Bragg, K.; et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N. Engl. J. Med. 1987, 317, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Fujisawa, M. Recovery of Spermatogenesis Following Cancer Treatment with Cytotoxic Chemotherapy and Radiotherapy. World J. Mens Health 2019, 37, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The impact of drugs on male fertility: A review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef]
- McCallum, T.; Milunsky, J.; Munarriz, R.; Carson, R.; Sadeghi-Nejad, H.; Oates, R. Unilateral renal agenesis associated with congenital bilateral absence of the vas deferens: Phenotypic findings and genetic considerations. Hum. Reprod. 2001, 16, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Mickle, J.; Milunsky, A.; Amos, J.A.; Oates, R.D. Congenital unilateral absence of the vas deferens: A heterogeneous disorder with two distinct subpopulations based upon aetiology and mutational status of the cystic fibrosis gene. Hum. Reprod. 1995, 10, 1728–1735. [Google Scholar] [CrossRef]
- Claustres, M. Molecular pathology of the CFTR locus in male infertility. Reprod. Biomed. Online 2005, 10, 14–41. [Google Scholar] [CrossRef]
- Costabile, R.A.; Spevak, M. Characterization of patients presenting with male factor infertility in an equal access, no cost medical system. Urology 2001, 58, 1021–1024. [Google Scholar] [CrossRef]
- Matsuda, T.; Horii, Y.; Yoshida, O. Unilateral obstruction of the vas deferens caused by childhood inguinal herniorrhaphy in male infertility patients. Fertil. Steril. 1992, 58, 609–613. [Google Scholar] [CrossRef]
- Shin, D.; Lipshultz, L.I.; Goldstein, M.; Barmé, G.A.; Fuchs, E.F.; Nagler, H.M.; McCallum, S.W.; Niederberger, C.S.; Schoor, R.A.; Brugh, V.M., 3rd; et al. Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: A preventable cause of obstructive azoospermia. Ann. Surg. 2005, 241, 553–558. [Google Scholar] [CrossRef]
- Farley, S.; Barnes, R. Stenosis of ejaculatory ducts treated by endoscopic resection. J. Urol. 1973, 109, 664–666. [Google Scholar] [CrossRef]
- Hopps, C.V.; Goldstein, M.; Schlegel, P.N. The diagnosis and treatment of the azoospermic patient in the age of intracytoplasmic sperm injection. Urol. Clin North. Am. 2002, 29, 895–911. [Google Scholar] [CrossRef]
- Pryor, J.P.; Hendry, W.F. Ejaculatory duct obstruction in subfertile males: Analysis of 87 patients. Fertil. Steril. 1991, 56, 725–730. [Google Scholar] [CrossRef]
- Schuster, T.G.; Ohl, D.A. Diagnosis and treatment of ejaculatory dysfunction. Urol. Clin North. Am. 2002, 29, 939–948. [Google Scholar] [CrossRef]
- Hendry, W.F. Disorders of ejaculation: Congenital, acquired and functional. Br. J. Urol. 1998, 82, 331–341. [Google Scholar] [CrossRef]
- López Andreu, J.A.; Fernández, P.J.; Ferrís i Tortajada, J.; Navarro, I.; Rodríguez-Ineba, A.; Antonio, P.; Muro, M.D.; Romeu, A. Persistent altered spermatogenesis in long-term childhood cancer survivors. Pediatr. Hematol. Oncol. 2000, 17, 21–30. [Google Scholar] [CrossRef]
- Rajfer, J. TESA or TESE: Which Is Better for Sperm Extraction? Rev. Urol. 2006, 8, 171. [Google Scholar] [PubMed]
- Ishikawa, H.; Takeshima, H. An evaluation of blood serotonin in infertile male patients. Hinyokika Kiyo 1984, 30, 1201–1205. [Google Scholar] [PubMed]
- Kanatsu-Shinohara, M.; Toyokuni, S.; Morimoto, T.; Matsui, S.; Honjo, T.; Shinohara, T. Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol. Reprod. 2003, 68, 1801–1807. [Google Scholar] [CrossRef]
- Ahn, J.S.; Ryu, H.-S.; Jung, S.-E.; Shin, B.-J.; Won, J.-H.; Um, T.G.; Oh, H.; Kim, S.-H.; Ryu, B.-Y. Expression profile of spermatogenesis associated genes in male germ cells during postnatal development in mice. J. Anim. Reprod. Biotechnol. 2020, 35, 289–296. [Google Scholar] [CrossRef]
- Han, N.R.; Park, H.J.; Lee, H.; Yun, J.I.; Choi, K.; Lee, E.; Lee, S.T. Identification of a Technique Optimized for the Isolation of Spermatogonial Stem Cells from Mouse Testes. J. Emb. Trans. 2018, 33, 327–336. [Google Scholar] [CrossRef]
- Valli, H.; Gassei, K.; Orwig, K.E. Stem Cell Therapies for Male Infertility: Where Are We Now and Where Are We Going? In Biennial Review of Infertility: Volume 4; Carrell, D.T., Schlegel, P.N., Racowsky, C., Gianaroli, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–39. [Google Scholar] [CrossRef]
- Forbes, C.M.; Flannigan, R.; Schlegel, P.N. Spermatogonial stem cell transplantation and male infertility: Current status and future directions. Arab. J. Urol. 2018, 16, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Geens, M.; Goossens, E.; De Block, G.; Ning, L.; Van Saen, D.; Tournaye, H. Autologous spermatogonial stem cell transplantation in man: Current obstacles for a future clinical application. Hum. Reprod. Update 2008, 14, 121–130. [Google Scholar] [CrossRef]
- Guan, K.; Nayernia, K.; Maier, L.S.; Wagner, S.; Dressel, R.; Lee, J.H.; Nolte, J.; Wolf, F.; Li, M.; Engel, W.; et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 2006, 440, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Takehashi, M.; Shinohara, T. Brief history, pitfalls, and prospects of mammalian spermatogonial stem cell research. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seandel, M.; James, D.; Shmelkov, S.V.; Falciatori, I.; Kim, J.; Chavala, S.; Scherr, D.S.; Zhang, F.; Torres, R.; Gale, N.W.; et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 2007, 449, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R Soc. Lond. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.E.; Park, M.H.; Kim, M.S.; Yun, J.I.; Choi, J.H.; Lee, E.; Lee, S.T. Development of a Three-dimensional Hydrogel System for the Maintenance of Porcine Spermatogonial Stem Cell Self-renewal. J. Emb. Trans. 2017, 32, 343–351. [Google Scholar] [CrossRef]
- Freeman, B. The active migration of germ cells in the embryos of mice and men is a myth. Reproduction 2003, 125, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Mollgard, K.; Jespersen, A.; Lutterodt, M.C.; Yding Andersen, C.; Hoyer, P.E.; Byskov, A.G. Human primordial germ cells migrate along nerve fibers and Schwann cells from the dorsal hind gut mesentery to the gonadal ridge. Mol. Hum. Reprod. 2010, 16, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Matte, R.; Sasaki, M. Autoradiographic evidence of human male germ-cell differentiation in vitro. Cytologia 1971, 36, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Cremades, N.; Bernabeu, R.; Barros, A.; Sousa, M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum. Reprod. 1999, 14, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Nagayoshi, M.; Awata, S.; Mawatari, Y.; Tanaka, I.; Kusunoki, H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil. Steril. 2003, 79 (Suppl 1), 795–801. [Google Scholar] [CrossRef]
- De Michele, F.; Poels, J.; Vermeulen, M.; Ambroise, J.; Gruson, D.; Guiot, Y.; Wyns, C. Haploid Germ Cells Generated in Organotypic Culture of Testicular Tissue from Prepubertal Boys. Front. Physiol. 2018, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Devolder, K. Complicity in stem cell research: The case of induced pluripotent stem cells. Hum. Reprod. 2010, 25, 2175–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef]
- Valli, H.; Phillips, B.T.; Orwig, K.E.; Gassei, K.; Nagano, M.C. Knobil and Neill’s Physiology of Reproduction, 4th ed.; Spermatogonial Stem Cells and Spermatogenesis; Academic Press: San Diego, CA, USA, 2015; pp. 595–635. [Google Scholar]
- Roosen-Runge, E.C. Quantitative studies on spermatogenesis in the albino rat. II. The duration of spermatogenesis and some effects of colchicine. Am. J. Anat. 1951, 88, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y.; Leblond, C.P. Renewal of spermatogonia in the rat. Am. J. Anat. 1953, 93, 475–501. [Google Scholar] [CrossRef]
- Huckins, C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat. Rec. 1971, 169, 533–557. [Google Scholar] [CrossRef]
- Oakberg, E.F. Spermatogonial stem-cell renewal in the mouse. Anat. Rec. 1971, 169, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Nabeshima, Y.; Nakagawa, T. Stem cell heterogeneity: Actual and potential stem cell compartments in mouse spermatogenesis. Ann. N. Y. Acad. Sci. 2007, 1120, 47–58. [Google Scholar] [CrossRef]
- Morimoto, H.; Kanatsu-Shinohara, M.; Takashima, S.; Chuma, S.; Nakatsuji, N.; Takehashi, M.; Shinohara, T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS ONE 2009, 4, e7909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huckins, C. The spermatogonial stem cell population in adult rats. II. A radioautographic analysis of their cell cycle properties. Cell Tissue Kinet 1971, 4, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Oakberg, E.F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am. J. Anat. 1956, 99, 391–413. [Google Scholar] [CrossRef] [PubMed]
- Waheeb, R.; Hofmann, M.C. Human spermatogonial stem cells: A possible origin for spermatocytic seminoma. Int. J. Androl. 2011, 34, e296–e305. [Google Scholar] [CrossRef] [Green Version]
- Clermont, Y.; Bustos-Obregon, E. Re-examination of spermatogonial renewal in the rat by means of seminiferous tubules mounted “in toto”. Am. J. Anat. 1968, 122, 237–247. [Google Scholar] [CrossRef]
- Dym, M.; Clermont, Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am. J. Anat. 1970, 128, 265–282. [Google Scholar] [CrossRef]
- Clermont, Y.; Antar, M. Duration of the cycle of the seminiferous epithelium and the spermatogonial renewal in the monkey Macaca arctoides. Am. J. Anat. 1973, 136, 153–165. [Google Scholar] [CrossRef]
- Kostereva, N.; Hofmann, M.C. Regulation of the Spermatogonial Stem Cell Niche. Reprod. Domest. Anim. 2008, 43, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Dadoune, J.P. New insights into male gametogenesis: What about the spermatogonial stem cell niche? Folia. Histochem. Cytobiol. 2007, 45, 141–147. [Google Scholar]
- De Rooij, D.G. The spermatogonial stem cell niche. Microsc. Res. Tech. 2009, 72, 580–585. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, D.G. Sertoli Cell Biology, 2nd ed.; The Spermatogonial Stem Cell Niche in Mammals; Academic Press: San Diego, CA, USA, 2015; pp. 99–121. [Google Scholar]
- Tung, P.S.; Skinner, M.K.; Fritz, I.B. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann. N. Y. Acad. Sci. 1984, 438, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Ogura, A.; Toyokuni, S.; Shinohara, T. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum. Reprod. 2003, 18, 2660–2667. [Google Scholar] [CrossRef] [Green Version]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Germline niche transplantation restores fertility in infertile mice. Hum. Reprod. 2005, 20, 2376–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.Y.; Mruk, D.D. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002, 82, 825–874. [Google Scholar] [CrossRef] [Green Version]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, M.; Hildorf, S.; Dong, L.; Thorup, J.; Hoffmann, E.R.; Jensen, C.F.S.; Sønksen, J.; Cortes, D.; Fedder, J.; Andersen, C.Y.; et al. Review of injection techniques for spermatogonial stem cell transplantation. Hum. Reprod. Update 2020, 26, 368–391. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.P.; Carlson, C.A.; Lin, K.; Hobbie, W.L.; Wigo, E.; Wu, X.; Brinster, R.L.; Kolon, T.F. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: A report of acceptability and safety. Hum. Reprod. 2010, 25, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.J.; Sung, S.Y.; Kim, H.J.; Song, S.H.; Hong, J.Y.; Yoon, T.K.; Kim, J.K.; Kim, K.S.; Lee, D.R. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010, 43, 405–417. [Google Scholar] [CrossRef]
- Oatley, J. Cryopreserving and Thawing Spermatogonial Stem Cells. Cold Spring Harb. Protoc. 2017, 2017, pdb.prot094219. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, S.; Brinster, R. Isolation of the Spermatogonial Stem Cell-Containing Fraction from Testes. Cold Spring Harb. Protoc. 2017, 2017, pdb.prot094185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, H.; Brinster, R.L. Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 2008, 86, 59–84. [Google Scholar] [CrossRef] [Green Version]
- de Siqueira-Silva, D.H.; Dos Santos Silva, A.P.; da Silva Costa, R.; Senhorini, J.A.; Ninhaus-Silveira, A.; Veríssimo-Silveira, R. Preliminary study on testicular germ cell isolation and transplantation in an endangered endemic species Brycon orbignyanus (Characiformes: Characidae). Fish. Physiol. Biochem. 2019, 3, 767–776. [Google Scholar] [CrossRef]
- Goodyear, S.; Brinster, R. Culture and Expansion of Primary Undifferentiated Spermatogonial Stem Cells. Cold Spring Harb. Protoc. 2017, 2017, pdb.prot094193. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.; Xiong, T.; Tang, W. Isolation and characterization of human spermatogonial stem cells. Reprod. Biol. Endocrinol. 2011, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Mohaqiq, M.; Movahedin, M.; Mazaheri, Z.; Amirjannati, N. In vitro transplantation of spermatogonial stem cells isolated from human frozen–thawed testis tissue can induce spermatogenesis under 3-dimensional tissue culture conditions. Biol. Res. 2019, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.A.; Seandel, M. Propagation of Adult SSCs: From Mouse to Human. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Voigt, A.L.; Thiageswaran, S.; de Lima e Martins Lara, N.; Dobrinski, I. Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 1998. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.Y.; Park, Y.S.; Ryu, J.-S.; Lee, H.J.; Araúzo-Bravo, M.J.; Ko, K.; Han, D.W.; Schöler, H.R.; Ko, K. A Novel Feeder-Free Culture System for Expansion of Mouse Spermatogonial Stem Cells. Mol. Cells 2014, 37, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lin, Q.; Zhou, C.; Li, Q.; Li, Z.; Cao, Z.; Liang, J.; Li, H.; Mei, J.; Zhang, Q.; et al. A Testis-Derived Hydrogel as an Efficient Feeder-Free Culture Platform to Promote Mouse Spermatogonial Stem Cell Proliferation and Differentiation. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, L.; Sun, M.; Hai, Y.; Li, Z.; He, Z. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp. Biol. Med. 2015, 240, 1112–1122. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Kokkinaki, M.; Jiang, J.; Dobrinski, I.; Dym, M. Isolation, Characterization, and Culture of Human Spermatogonia1. Biol. Reprod. 2010, 82, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Niu, M.; Liu, L.; Zhu, Z.; Wang, X.; Sun, M.; Yuan, Q.; Yang, S.; Zeng, W.; Liu, Y.; et al. Establishment and Characterization of Human Germline Stem Cell Line with Unlimited Proliferation Potentials and no Tumor Formation. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Cui, Y.; Liu, B.; Li, C.; Du, L.; Tang, R.; Qin, L.; Jiang, Y.; Li, J.; Yu, X.; et al. Hsa-miR-1908-3p Mediates the Self-Renewal and Apoptosis of Human Spermatogonial Stem Cells via Targeting KLF2. Mol. Ther. Nucleic Acids 2020, 20, 788–800. [Google Scholar] [CrossRef]
- Nagano, M.; Patrizio, P.; Brinster, R.L. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 2002, 78, 1225–1233. [Google Scholar] [CrossRef]
- Mohaqiq, M.; Movahedin, M.; Mazaheri, Z.; Amirjannati, N. Successful Human Spermatogonial Stem Cells Homing in Recipient Mouse Testis after In Vitro Transplantation and Organ Culture. Cell J. 2019, 20, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Turner, D.; Nelson, J.; Dobrinski, I.; McEntee, M.; Travis, A.J. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction 2008, 136, 823–831. [Google Scholar] [CrossRef]
- Shetty, G.; Mitchell, J.M.; Lam, T.N.A.; Phan, T.T.; Zhang, J.; Tailor, R.C.; Peters, K.A.; Penedo, M.C.; Hanna, C.B.; Clark, A.T.; et al. Postpubertal Spermatogonial Stem Cell Transplantation Restores Functional Sperm Production in Rhesus Monkeys Irradiated Before and After Puberty. Andrology 2021. [CrossRef]
- Eildermann, K.; Gromoll, J.; Behr, R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum. Reprod. 2012, 27, 1754–1767. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kang, H.G.; Kim, B.J.; Jung, S.E.; Karmakar, P.C.; Kim, S.M.; Hwang, S.; Ryu, B.Y. Enrichment and In Vitro Culture of Spermatogonial Stem Cells from Pre-Pubertal Monkey Testes. Tissue Eng Regen Med. 2017, 14, 557–566. [Google Scholar] [CrossRef]
- Trowell, O.A. The culture of mature organs in a synthetic medium. Exp. Cell Res. 1959, 16, 118–147. [Google Scholar] [CrossRef]
- Steinberger, E.; Steinberger, A.; Perloff, W.H. Initiation of spermatogenesis in vitro. Endocrinology 1964, 74, 788–792. [Google Scholar] [CrossRef]
- Steinberger, E.; Steinberger, A.; Perloff, W.H. Studies on growth in organ culture of testicular tissue from rats of various ages. Anat. Rec. 1964, 148, 581–589. [Google Scholar] [CrossRef]
- Boitani, C.; Politi, M.G.; Menna, T. Spermatogonial cell proliferation in organ culture of immature rat testis. Biol. Reprod. 1993, 48, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sato, K. The fertilising ability of spermatogenic cells derived from cultured mouse immature testicular tissue. Zygote 2003, 11, 307–316. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yokonishi, T.; Komeya, M.; Katagiri, K.; Kubota, Y.; Matoba, S.; Ogonuki, N.; Ogura, A.; Yoshida, S.; Ogawa, T. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc. Natl. Acad. Sci. USA 2012, 109, 16934–16938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokonishi, T.; Sato, T.; Komeya, M.; Katagiri, K.; Kubota, Y.; Nakabayashi, K.; Hata, K.; Inoue, K.; Ogonuki, N.; Ogura, A.; et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun. 2014, 5, 4320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Nie, J.; Zhu, X.; Lu, Y.; Liang, X.; Xu, H.; Yang, X.; Zhang, Y.; Lu, K.; Lu, S. In vitro differentiation of spermatogonial stem cells using testicular cells from Guangxi Bama mini-pig. J Vet Sci. 2018, 19, 592–599. [Google Scholar] [CrossRef]
- Ibtisham, F.; Honaramooz, A. Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Dumont, L.; Oblette, A.; Rondanino, C.; Jumeau, F.; Bironneau, A.; Liot, D.; Duchesne, V.; Wils, J.; Rives, N. Vitamin A prevents round spermatid nuclear damage and promotes the production of motile sperm during in vitro maturation of vitrified pre-pubertal mouse testicular tissue. Mol. Hum. Reprod. 2016, 22, 819–832. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, T.; Sato, T.; Abe, T.; Sanjo, H.; Katagiri, K.; Kimura, H.; Fujii, T.; Tanaka, H.; Hirabayashi, M.; Ogawa, T. Rat in vitro spermatogenesis promoted by chemical supplementations and oxygen-tension control. Sci. Rep. 2021, 11, 3458. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, M.; Jorsaraei, S.G.A.; Fattahi, E.; Tabari, M.G.; Neyshaburi, E.Z. SCF Improves In Vitro Differentiation of SSCs Through Transcriptionally Up-regulating PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 Genes. Reprod. Sci. 2021, 28, 963–972. [Google Scholar] [CrossRef]
- Arkoun, B.; Galas, L.; Dumont, L.; Rives, A.; Saulnier, J.; Delessard, M.; Rondanino, C.; Rives, N. Vitamin E but Not GSH Decreases Reactive Oxygen Species Accumulation and Enhances Sperm Production during In Vitro Maturation of Frozen-Thawed Prepubertal Mouse Testicular Tissue. Int. J. Mol. Sci. 2019, 20, 5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurkure, P.; Prasad, M.; Dhamankar, V.; Bakshi, G. Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod. Biol. Endocrinol. 2015, 13, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update 2021, 27, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, M.; Tian, R.; Wang, Y.; Liu, L.; Zhu, Z.; Yang, S.; Yuan, Q.; Niu, M.; Yao, C.; et al. Derivation and propagation of spermatogonial stem cells from human pluripotent cells. Stem Cell Res. Ther. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Cakici, C.; Buyrukcu, B.; Duruksu, G.; Haliloglu, A.H.; Aksoy, A.; Isık, A.; Uludag, O.; Ustun, H.; Subası, C.; Karaoz, E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed Res. Int. 2013, 2013, 529589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Peng, J.; He, D.; Lin, T.; Zhu, J.; Li, X.; Zhang, Y.; Wei, G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int. J. Mol. Sci. 2014, 15, 13151–13165. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.; Ntemou, E.; Onofre, J.; Van Saen, D.; Goossens, E. Does co-transplantation of mesenchymal and spermatogonial stem cells improve reproductive efficiency and safety in mice? Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]


| Species | Level of In Vitro Culture Achieved | Time of In Vitro Culture (Year Achieved) | Culture Media Used | Reference |
|---|---|---|---|---|
| Human | Testicular tissue maintained | Several weeks (1970) | Eagle’ss minimum essential media (MEM) | [92] |
| Propagation of SSCs but in undifferentiated | Two months propagation (2015) | StemPro-34 SFM (serum free medium) | [137] | |
| Monkey | Maintained SSCs | Only short time (2012) | Germline culture medium | [145] |
| Maintained SSCs | Effective for longer time (2017) | Stem-Pro medium | [146] | |
| Mouse | Organ (testicular) fragment maintained | 6 days (1959) | Eagle’ss MEM | [147] |
| Organ (testicular) fragment maintained | 4 weeks (1964) | Eagle’ss MEM | [148] | |
| Organ maintained and differentiation of spermatogonia to spermatocytes | 2–3 weeks of culture (1964) | Eagle’ss MEM | [149] | |
| Differentiation of type A spermatogonia into meiotic pachytene spermatocytes | After 3 weeks (1993) | FSH supplemented Eagle’ss MEM | [150] | |
| Round spermatids observed (able to fertilize oocyte) | After 2 weeks of culture (2003) | Gas-liquid interface culture system | [151] | |
| Obtained spermatid and sperm (Produced reproductively competent offspring by microinsemination) | After 2 months (2011) [the first successful IVS] | Gas-liquid interface culture system (serum free) | [152] | |
| Haploid male germ cells obtained from infertile mutant mouse (Offspring produced) | Monitored growth (2012) | Agarose gel in α-MEM supplemented with KO serum replacement (KSR) or AlbuMAX | [153] | |
| IVS also achieved from cryopreserved testis tissue (Offspring produced) | Monitored growth (2014) | Agarose gel in α-MEM supplemented with KSR or AlbuMAX | [154] | |
| Minipig | SSCs differentiate and develop to a post-meiotic stage | Ten days culture (2018) | MEM-α supplemented with KO serum replacement | [155] |
| Subjects/Cases | Intervention | Outcome | Geographic Location | Status | Trial Identifier |
|---|---|---|---|---|---|
| Azoospermic Patients | Bone Marrow Derived Mesenchymal Stem Cells | No results posted | Cairo, Egypt | Recruiting | NCT02025270 |
| Non-obstructive Azoospermia | Bone marrow derived CD34+, CD133+, and mesenchymal stem cells | No results posted | Amman, Jordan | Recruiting | NCT02641769 |
| Klinefelter Syndrome Azoospermia | Bone marrow Mesenchymal stem cell injection | No results posted | Cairo, Egypt | Recruiting | NCT02414295 |
| Non-obstructive Azoospermia | Bone Marrow Derived Stem Cells | No results posted | Giza, Egypt | Recruiting | NCT02041910 |
| Non-obstructive Azoospermia | Bone Marrow Derived Stem Cells | No results posted | Cairo, Egypt | Recruiting | NCT02008799 |
| Azoospermia and oligozoospermia | Adipose-Derived Adult Stromal Vascular Cells | No results posted | Samara, Russian Federation | Enrolling by invitation | NCT03762967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, N.E.; Tanga, B.M.; Abdelgawad, M.; Allam, S.; Fathi, M.; Saadeldin, I.M.; Bang, S.; Cho, J. Cellular Therapy via Spermatogonial Stem Cells for Treating Impaired Spermatogenesis, Non-Obstructive Azoospermia. Cells 2021, 10, 1779. https://doi.org/10.3390/cells10071779
Abdelaal NE, Tanga BM, Abdelgawad M, Allam S, Fathi M, Saadeldin IM, Bang S, Cho J. Cellular Therapy via Spermatogonial Stem Cells for Treating Impaired Spermatogenesis, Non-Obstructive Azoospermia. Cells. 2021; 10(7):1779. https://doi.org/10.3390/cells10071779
Chicago/Turabian StyleAbdelaal, Nesma E., Bereket Molla Tanga, Mai Abdelgawad, Sahar Allam, Mostafa Fathi, Islam M. Saadeldin, Seonggyu Bang, and Jongki Cho. 2021. "Cellular Therapy via Spermatogonial Stem Cells for Treating Impaired Spermatogenesis, Non-Obstructive Azoospermia" Cells 10, no. 7: 1779. https://doi.org/10.3390/cells10071779
APA StyleAbdelaal, N. E., Tanga, B. M., Abdelgawad, M., Allam, S., Fathi, M., Saadeldin, I. M., Bang, S., & Cho, J. (2021). Cellular Therapy via Spermatogonial Stem Cells for Treating Impaired Spermatogenesis, Non-Obstructive Azoospermia. Cells, 10(7), 1779. https://doi.org/10.3390/cells10071779