Stem Cell Application in Infertility
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 78178
Share This Topical Collection
Editors
 Prof. Dr. Eunju Kang
Prof. Dr. Eunju Kang
 Prof. Dr. Eunju Kang
Prof. Dr. Eunju Kang
E-Mail
Website
Collection Editor
1. Associate Professor, Department of Biochemistry, School of Medicine & Biomedical Science, CHA University, Seongnam-si 13488, Gyeonggi-do, Republic of Korea
2. Director, Cell Therapy 3 Center, CHA Advanced Research Institute, CHA Bundang Medical Center, Sungnam-si 13488, Gyeonggi-do, Republic of Korea
Interests: pluripotent stem cells; hematopoietic stem cells; immune cells; megakaryocytes
Special Issues, Collections and Topics in MDPI journals
 Prof. Dr. Jongki Cho
Prof. Dr. Jongki Cho
 Prof. Dr. Jongki Cho
Prof. Dr. Jongki Cho
E-Mail
Collection Editor
College of Veterinary Medicine, Chungnam National University, 99, Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
Interests: stem cells; animal; reproduction; cloning
Topical Collection Information
Dear Colleagues,
Assisted reproductive technologies (ART) such as in-vitro fertilization (IVF) have overcome several types of infertility. Nevertheless, about 10% of couples suffer from infertility due to problems with the female reproductive system, insufficient production of gametes, or the poor ability of sperm or oocytes.
Recently, attempts have been made to treat the problems with the female reproductive system in clinics using adult stem cells such as mesenchymal stem cells (MSCs) or hematopoietic stem cells (HSCs). The results were promising, but no clear mechanism has been investigated.
Further, embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) could be converted into germ cells such as sperm or oocytes. However, a few laboratories can perform this technology.
This Topical Collection focuses on therapeutic mechanisms for stem cell application in the field of infertility, including basic, preclinical and clinical studies, and the replication of current technologies.
We look forward to your contributions.
Prof. Dr. Eunju Kang
Prof. Dr. Jongki Cho
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Mesenchymal stem cell for infertility
- Hematopoietic stem cell for infertility
- Artificial sperm
- Artificial oocytes
- Germ cell differentiation
Published Papers (9 papers)
Open AccessReview
Artificial Oocyte: Development and Potential Application
by
Reza K. Oqani, Seongjun So, Yeonmi Lee, Jung Jae Ko and Eunju Kang
Cited by 7 | Viewed by 9622
Abstract
Millions of people around the world suffer from infertility, with the number of infertile couples and individuals increasing every year. Assisted reproductive technologies (ART) have been widely developed in recent years; however, some patients are unable to benefit from these technologies due to
[...] Read more.
Millions of people around the world suffer from infertility, with the number of infertile couples and individuals increasing every year. Assisted reproductive technologies (ART) have been widely developed in recent years; however, some patients are unable to benefit from these technologies due to their lack of functional germ cells. Therefore, the development of alternative methods seems necessary. One of these methods is to create artificial oocytes. Oocytes can be generated in vitro from the ovary, fetal gonad, germline stem cells (GSCs), ovarian stem cells, or pluripotent stem cells (PSCs). This approach has raised new hopes in both basic research and medical applications. In this article, we looked at the principle of oocyte development, the landmark studies that enhanced our understanding of the cellular and molecular mechanisms that govern oogenesis in vivo, as well as the mechanisms underlying in vitro generation of functional oocytes from different sources of mouse and human stem cells. In addition, we introduced next-generation ART using somatic cells with artificial oocytes. Finally, we provided an overview of the reproductive application of in vitro oogenesis and its use in human fertility.
Full article
►▼
Show Figures
Open AccessReview
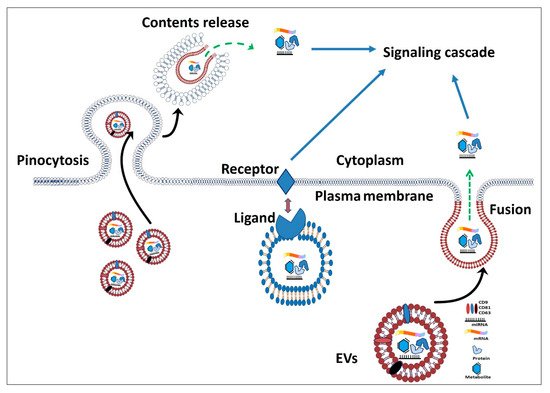
The Role of Stem Cells and Their Derived Extracellular Vesicles in Restoring Female and Male Fertility
by
Ahmad Yar Qamar, Tariq Hussain, Muhammad Kamran Rafique, Seonggyu Bang, Bereket Molla Tanga, Gyeonghwan Seong, Xun Fang, Islam M. Saadeldin and Jongki Cho
Cited by 14 | Viewed by 5501
Abstract
Infertility is a globally recognized issue caused by different reproductive disorders. To date, various therapeutic approaches to restore fertility have been attempted including etiology-specific medication, hormonal therapies, surgical excisions, and assisted reproductive technologies. Although these approaches produce results, however, fertility restoration is not
[...] Read more.
Infertility is a globally recognized issue caused by different reproductive disorders. To date, various therapeutic approaches to restore fertility have been attempted including etiology-specific medication, hormonal therapies, surgical excisions, and assisted reproductive technologies. Although these approaches produce results, however, fertility restoration is not achieved in all cases. Advances in using stem cell (SC) therapy hold a great promise for treating infertile patients due to their abilities to self-renew, differentiate, and produce different paracrine factors to regenerate the damaged or injured cells and replenish the affected germ cells. Furthermore, SCs secrete extracellular vesicles (EVs) containing biologically active molecules including nucleic acids, lipids, and proteins. EVs are involved in various physiological and pathological processes and show promising non-cellular therapeutic uses to combat infertility. Several studies have indicated that SCs and/or their derived EVs transplantation plays a crucial role in the regeneration of different segments of the reproductive system, oocyte production, and initiation of sperm production. However, available evidence triggers the need to testify the efficacy of SC transplantation or EVs injection in resolving the infertility issues of the human population. In this review, we highlight the recent literature covering the issues of infertility in females and males, with a special focus on the possible treatments by stem cells or their derived EVs.
Full article
►▼
Show Figures
Open AccessArticle
Analysis of the Reversible Impact of the Chemodrug Busulfan on Mouse Testes
by
Laura Thirouard, Hélène Holota, Mélusine Monrose, Manon Garcia, Angélique De Haze, Jean-Paul Saru, Françoise Caira, Claude Beaudoin and David H. Volle
Cited by 7 | Viewed by 2621
Abstract
Spermatogenesis is a process within the testis that leads to the production of spermatozoa. It is based on a population of spermatogonial stem cells, which have the capacity to self-renew and to differentiate throughout life to ensure the functions of reproduction are maintained.
[...] Read more.
Spermatogenesis is a process within the testis that leads to the production of spermatozoa. It is based on a population of spermatogonial stem cells, which have the capacity to self-renew and to differentiate throughout life to ensure the functions of reproduction are maintained. Male fertility disorders are responsible for half of the cases of infertility in couples worldwide. It is well known that cancer treatments are associated with reversible or irreversible fertility disorders. Busulfan (Bu) is an alkylating agent that significantly inhibits spermatogenesis. The present study relied on a combination of in vivo and in vitro approaches as well as RNAseq analysis to characterize the effects of Bu, in which mouse testes were used as a model. An in silico analysis revealed that many of the Bu-modulated genes are potentially regulated by the SIN3 Transcription Regulator Family Member A (SIN3A) and E2F Transcription Factor (E2F) families of transcription factors. The results demonstrate that the deregulated genes function in processes related to the cell cycle, DNA repair, and cell death mechanisms, including the Tumor Protein 53 (TP53) pathway. This reinforces the role of the TP53 signaling pathway as a major player in Bu effects. In addition, Bu altered the patterns of mRNA accumulation for various genes in undifferentiated spermatogonia. This work provides significant insight into the kinetics and impacts of busulfan, which could pave the way for developing strategies to minimize the impact of chemodrugs and, thus, could lead to germ cell lineage regeneration following anticancer treatments.
Full article
►▼
Show Figures
Open AccessReview
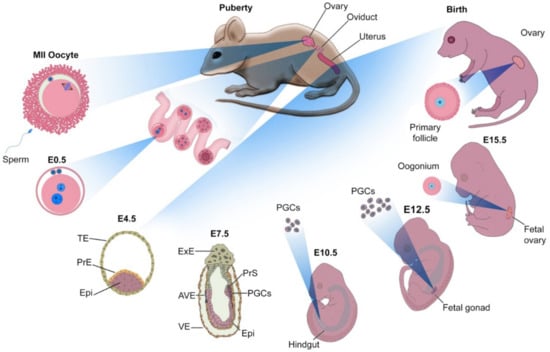
Germ Cell Derivation from Pluripotent Stem Cells for Understanding In Vitro Gametogenesis
by
Tae-Kyung Hong, Jae-Hoon Song, So-Been Lee and Jeong-Tae Do
Cited by 10 | Viewed by 11394
Abstract
Assisted reproductive technologies (ARTs) have developed considerably in recent years; however, they cannot rectify germ cell aplasia, such as non-obstructive azoospermia (NOA) and oocyte maturation failure syndrome. In vitro gametogenesis is a promising technology to overcome infertility, particularly germ cell aplasia. Early germ
[...] Read more.
Assisted reproductive technologies (ARTs) have developed considerably in recent years; however, they cannot rectify germ cell aplasia, such as non-obstructive azoospermia (NOA) and oocyte maturation failure syndrome. In vitro gametogenesis is a promising technology to overcome infertility, particularly germ cell aplasia. Early germ cells, such as primordial germ cells, can be relatively easily derived from pluripotent stem cells (PSCs); however, further progression to post-meiotic germ cells usually requires a gonadal niche and signals from gonadal somatic cells. Here, we review the recent advances in in vitro male and female germ cell derivation from PSCs and discuss how this technique is used to understand the biological mechanism of gamete development and gain insight into its application in infertility.
Full article
►▼
Show Figures
Open AccessReview
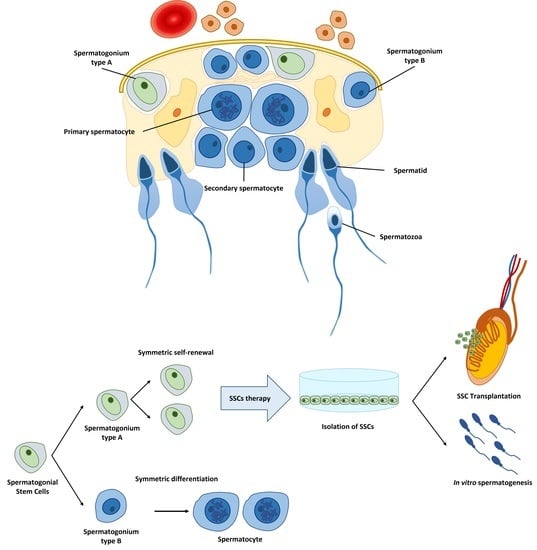
Cellular Therapy via Spermatogonial Stem Cells for Treating Impaired Spermatogenesis, Non-Obstructive Azoospermia
by
Nesma E. Abdelaal, Bereket Molla Tanga, Mai Abdelgawad, Sahar Allam, Mostafa Fathi, Islam M. Saadeldin, Seonggyu Bang and Jongki Cho
Cited by 20 | Viewed by 10783
Abstract
Male infertility is a major health problem affecting about 8–12% of couples worldwide. Spermatogenesis starts in the early fetus and completes after puberty, passing through different stages. Male infertility can result from primary or congenital, acquired, or idiopathic causes. The absence of sperm
[...] Read more.
Male infertility is a major health problem affecting about 8–12% of couples worldwide. Spermatogenesis starts in the early fetus and completes after puberty, passing through different stages. Male infertility can result from primary or congenital, acquired, or idiopathic causes. The absence of sperm in semen, or azoospermia, results from non-obstructive causes (pretesticular and testicular), and post-testicular obstructive causes. Several medications such as antihypertensive drugs, antidepressants, chemotherapy, and radiotherapy could lead to impaired spermatogenesis and lead to a non-obstructive azoospermia. Spermatogonial stem cells (SSCs) are the basis for spermatogenesis and fertility in men. SSCs are characterized by their capacity to maintain the self-renewal process and differentiation into spermatozoa throughout the male reproductive life and transmit genetic information to the next generation. SSCs originate from gonocytes in the postnatal testis, which originate from long-lived primordial germ cells during embryonic development. The treatment of infertility in males has a poor prognosis. However, SSCs are viewed as a promising alternative for the regeneration of the impaired or damaged spermatogenesis. SSC transplantation is a promising technique for male infertility treatment and restoration of spermatogenesis in the case of degenerative diseases such as cancer, radiotherapy, and chemotherapy. The process involves isolation of SSCs and cryopreservation from a testicular biopsy before starting cancer treatment, followed by intra-testicular stem cell transplantation. In general, treatment for male infertility, even with SSC transplantation, still has several obstacles. The efficiency of cryopreservation, exclusion of malignant cells contamination in cancer patients, and socio-cultural attitudes remain major challenges to the wider application of SSCs as alternatives. Furthermore, there are limitations in experience and knowledge regarding cryopreservation of SSCs. However, the level of infrastructure or availability of regulatory approval to process and preserve testicular tissue makes them tangible and accurate therapy options for male infertility caused by non-obstructive azoospermia, though in their infancy, at least to date.
Full article
►▼
Show Figures
Open AccessReview
Application of Stem Cell Therapy for Infertility
by
Sarama Saha, Partha Roy, Cynthia Corbitt and Sham S. Kakar
Cited by 51 | Viewed by 13515
Abstract
Infertility creates an immense impact on the psychosocial wellbeing of affected couples, leading to poor quality of life. Infertility is now considered to be a global health issue affecting approximately 15% of couples worldwide. It may arise from factors related to the male
[...] Read more.
Infertility creates an immense impact on the psychosocial wellbeing of affected couples, leading to poor quality of life. Infertility is now considered to be a global health issue affecting approximately 15% of couples worldwide. It may arise from factors related to the male (30%), including varicocele, undescended testes, testicular cancer, and azoospermia; the female (30%), including premature ovarian failure and uterine disorders; or both partners (30%). With the recent advancement in assisted reproduction technology (ART), many affected couples (80%) could find a solution. However, a substantial number of couples cannot conceive even after ART. Stem cells are now increasingly being investigated as promising alternative therapeutics in translational research of regenerative medicine. Tremendous headway has been made to understand the biology and function of stem cells. Considering the minimum ethical concern and easily available abundant resources, extensive research is being conducted on induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSC) for their potential application in reproductive medicine, especially in cases of infertility resulting from azoospermia and premature ovarian insufficiency. However, most of these investigations have been carried out in animal models. Evolutionary divergence observed in pluripotency among animals and humans requires caution when extrapolating the data obtained from murine models to safely apply them to clinical applications in humans. Hence, more clinical trials based on larger populations need to be carried out to investigate the relevance of stem cell therapy, including its safety and efficacy, in translational infertility medicine.
Full article
►▼
Show Figures
Open AccessArticle
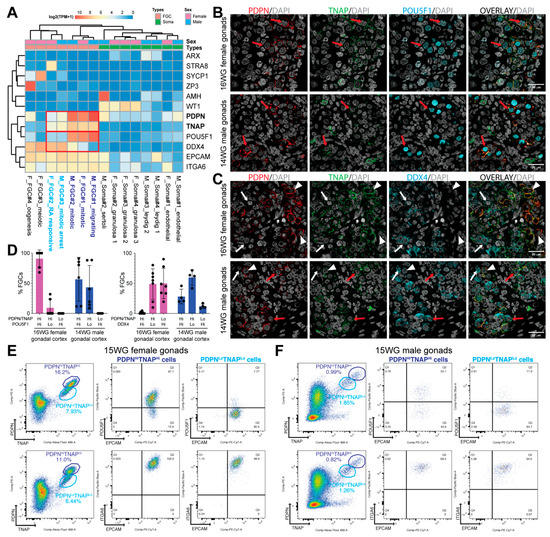
Sex-Specific Isolation and Propagation of Human Premeiotic Fetal Germ Cells and Germ Cell-Like Cells
by
Swati Mishra, Jasin Taelman, Yolanda W. Chang, Annekatrien Boel, Petra De Sutter, Björn Heindryckx and Susana M. Chuva De Sousa Lopes
Cited by 10 | Viewed by 3611
Abstract
The second trimester of human development is marked by asynchronous gonadal development hampering the isolation of homogenous populations of early and late fetal germ cells (FGCs). We evaluated the feasibility of using surface markers TNAP, PDPN, EPCAM and ITGA6 to isolate FGCs as
[...] Read more.
The second trimester of human development is marked by asynchronous gonadal development hampering the isolation of homogenous populations of early and late fetal germ cells (FGCs). We evaluated the feasibility of using surface markers TNAP, PDPN, EPCAM and ITGA6 to isolate FGCs as well as human primordial germ cell-like cells (hPGCLCs) derived from embryonic stem cells (hESCs) from both sexes by fluorescence-activated cell sorting (FACS). Our results suggest that a combination of TNAP and PDPN was sufficient to separate populations of premeiotic FGCs and hPGCLCs in both sexes. This combination of antibodies also proved efficient in separating ‘mitotic’ from ‘retinoic-acid responsive’ female FGCs. Furthermore, we report that the differentiation efficiency of TNAP+PDPN+ hPGCLCs from hESCs was sex-independent, but the ability to propagate differed considerably between the sexes. In contrast to male, female hPGCLCs retained their characteristics and exhibited robust colony-forming ability when cultured for five days in medium containing LIF, forskolin and FGF2. We conclude that marked sex differences exist in the isolation and propagation of human FGCs and hPGCLCs. Our study provides novel insights relevant for the optimization of in vitro gametogenesis in humans.
Full article
►▼
Show Figures
Open AccessReview
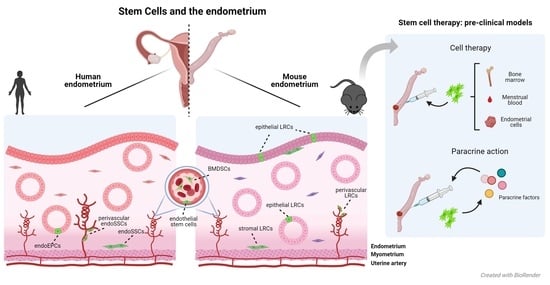
Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models
by
Lucía de Miguel-Gómez, Sara López-Martínez, Emilio Francés-Herrero, Adolfo Rodríguez-Eguren, Antonio Pellicer and Irene Cervelló
Cited by 30 | Viewed by 8016
Abstract
Adult stem cells (ASCs) were long suspected to exist in the endometrium. Indeed, several types of endometrial ASCs were identified in rodents and humans through diverse isolation and characterization techniques. Putative stromal and epithelial stem cell niches were identified in murine models using
[...] Read more.
Adult stem cells (ASCs) were long suspected to exist in the endometrium. Indeed, several types of endometrial ASCs were identified in rodents and humans through diverse isolation and characterization techniques. Putative stromal and epithelial stem cell niches were identified in murine models using label-retention techniques. In humans, functional methods (clonogenicity, long-term culture, and multi-lineage differentiation assays) and stem cell markers (CD146, SUSD2/W5C5, LGR5, NTPDase2, SSEA-1, or N-cadherin) facilitated the identification of three main types of endogenous endometrial ASCs: stromal, epithelial progenitor, and endothelial stem cells. Further, exogenous populations of stem cells derived from bone marrow may act as key effectors of the endometrial ASC niche. These findings are promoting the development of stem cell therapies for endometrial pathologies, with an evolution towards paracrine approaches. At the same time, promising therapeutic alternatives based on bioengineering have been proposed.
Full article
►▼
Show Figures
Open AccessReview
Mesenchymal Stem Cells as a Bio Organ for Treatment of Female Infertility
by
Sahar Esfandyari, Rishi Man Chugh, Hang-soo Park, Elie Hobeika, Mara Ulin and Ayman Al-Hendy
Cited by 71 | Viewed by 11025
Abstract
Female infertility is a global medical condition that can be caused by various disorders of the reproductive system, including premature ovarian failure (POF), polycystic ovary syndrome (PCOS), endometriosis, Asherman syndrome, and preeclampsia. It affects the quality of life of both patients and couples.
[...] Read more.
Female infertility is a global medical condition that can be caused by various disorders of the reproductive system, including premature ovarian failure (POF), polycystic ovary syndrome (PCOS), endometriosis, Asherman syndrome, and preeclampsia. It affects the quality of life of both patients and couples. Mesenchymal stem cells (MSCs) have received increasing attention as a potential cell-based therapy, with several advantages over other cell sources, including greater abundance, fewer ethical considerations, and high capacity for self-renewal and differentiation. Clinical researchers have examined the therapeutic use of MSCs in female infertility. In this review, we discuss recent studies on the use of MSCs in various reproductive disorders that lead to infertility. We also describe the role of microRNAs (miRNAs) and exosomal miRNAs in controlling MSC gene expression and driving MSC therapeutic outcomes. The clinical application of MSCs holds great promise for the treatment of infertility or ovarian insufficiency, and to improve reproductive health for a significant number of women worldwide.
Full article
►▼
Show Figures