Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance
Abstract
:1. Introduction
2. Subcellular Localization of HSP90
2.1. Cytosolic Localization of HSP90
2.2. Nuclear Localization of HSP90
2.3. Mitochondrial Localization of HSP90
2.4. Membrane and Extracellular Localization of HSP90
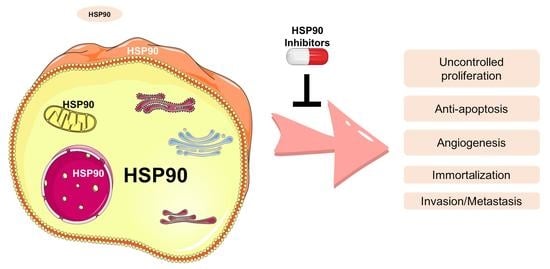
3. HSP90 and Its Clients in Cancer Phenotype
3.1. Uncontrolled Proliferation
3.2. Anti-Apoptosis and Immortalization
3.3. Invasion, Metastasis, and Angiogenic Role
3.4. Others
4. HSP90 Inhibitors in Cancer Therapeutics
4.1. HSP90 Inhibitors That Directly Bind to HSP90
4.1.1. Geldanamycin (GA) and Its Derivatives
4.1.2. Radicicol and Its Derivatives
4.1.3. Chimeric Molecules
4.1.4. Purine-Based Molecules
4.1.5. FDA-Approved Inhibitors Target HSP90
4.1.6. Other Inhibitors
4.2. Inhibitors of HSP90 Co-Chaperones and Clients
4.3. Nanoparticles for HSP90 Inhibitors Delivery
4.4. Mechanism of Resistance to HSP90 Inhibitors
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Chin, N.; Stanek, A.; Keh, W.; Lanks, K. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol. Cell. Biol. 1984, 4, 2802–2810. [Google Scholar] [PubMed]
- Borkovich, K.; Farrelly, F.; Finkelstein, D.; Taulien, J.; Lindquist, S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 1989, 9, 3919–3930. [Google Scholar] [PubMed]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohaszka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Buchner, J.; Li, J. Structure, Function and Regulation of the Hsp90 Machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [CrossRef]
- Blagg, B.S.J.; Kerr, T.D. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med. Res. Rev. 2006, 26, 310–338. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative Analysis of Hsp90-Client Interactions Reveals Principles of Substrate Recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef] [Green Version]
- Zuehlke, A.; Johnson, J.L. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 2009, 93, 211–217. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Drysdale, M.J.; Brough, P.A.; Massey, A.; Jensen, M.R.; Schoepfer, J. Targeting Hsp90 for the treatment of cancer. Curr. Opin. Drug Discov. Dev. 2006, 9, 483–495. [Google Scholar]
- Münster, P.N.; Marchion, D.C.; Basso, A.D.; Rosen, N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res. 2002, 62, 3132–3137. [Google Scholar]
- Terasawa, K.; Minami, M.; Minami, Y. Constantly Updated Knowledge of Hsp90. J. Biochem. 2005, 137, 443–447. [Google Scholar] [CrossRef]
- Holt, S.E.; Aisner, D.L.; Baur, J.; Tesmer, V.M.; Dy, M.; Ouellette, M.; Trager, J.B.; Morin, G.B.; Toft, D.O.; Shay, J.W.; et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999, 13, 817–826. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Beebe, K.; Neckers, L. Impact of heat-shock protein 90 on cancer metastasis. Futur. Oncol. 2009, 5, 679–688. [Google Scholar] [CrossRef]
- Hong, D.S.; Banerji, U.; Tavana, B.; George, G.C.; Aaron, J.; Kurzrock, R. Targeting the molecular chaperone heat shock protein 90 (HSP90): Lessons learned and future directions. Cancer Treat. Rev. 2012, 39, 375–387. [Google Scholar] [CrossRef]
- Nardai, G.; Schnaider, T.; Söti, C.; Ryan, M.; Hoj, P.B.; Somogyi, J.; Csermely, P. Characterization of the 90 kDa heat shock protein (HSP90)-associated ATP/GTPase. J. Biosci. 1996, 21, 179–190. [Google Scholar] [CrossRef]
- Passinen, S.; Valkila, J.; Manninen, T.; Syvälä, H.; Ylikomi, T. The C-terminal half of Hsp90 is responsible for its cytoplasmic localization. JBIC J. Biol. Inorg. Chem. 2001, 268, 5337–5342. [Google Scholar] [CrossRef]
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004, 5, 781–791. [Google Scholar] [CrossRef]
- Vermeulen, K.; Naus, E.; Ahamed, M.; Attili, B.; Siemons, M.; Luyten, K.; Celen, S.; Schymkowitz, J.; Rousseau, F.; Bormans, G. Evaluation of [11C]NMS-E973 as a PET tracer for in vivo visualisation of HSP90. Theranostics 2019, 9, 554–572. [Google Scholar] [CrossRef]
- Milicevic, Z.; Bogojevic, D.; Mihailovic, M.; Petrovic, M.; Krivokapic, Z. Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int. J. Oncol. 2008, 32, 1169–1178. [Google Scholar]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef]
- Ferrarini, M.; Heltai, S.; Zocchi, M.R.; Rugarli, C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J. Cancer 1992, 51, 613–619. [Google Scholar] [CrossRef]
- Kang, K.I.; Devin, J.; Cadepond, F.; Jibard, N.; Guiochon-Mantel, A.; Baulieu, E.E.; Catelli, M.G. In vivo functional protein-protein interaction: Nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc. Natl. Acad. Sci. USA 1994, 91, 340–344. [Google Scholar] [CrossRef]
- Koyasu, S.; Nishida, E.; Kadowaki, T.; Matsuzaki, F.; Iida, K.; Harada, F.; Kasuga, M.; Sakai, H.; Yahara, I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 8054–8058. [Google Scholar] [CrossRef]
- Weis, F.; Moullintraffort, L.; Heichette, C.; Chrétien, D.; Garnier, C. The 90-kDa Heat Shock Protein Hsp90 Protects Tubulin against Thermal Denaturation. J. Biol. Chem. 2010, 285, 9525–9534. [Google Scholar] [CrossRef]
- Etard, C.; Roostalu, U.; Strähle, U. Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J. Cell Biol. 2008, 180, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed]
- Diehl, M.C.; Idowu, M.O.; Kimmelshue, K.; York, T.P.; Elmore, L.W.; Holt, S.E. Elevated expression of nuclear Hsp90 in invasive breast tumors. Cancer Biol. Ther. 2009, 8, 1952–1961. [Google Scholar] [CrossRef] [Green Version]
- Su, J.-M.; Hsu, Y.-Y.; Lin, P.; Chang, H. Nuclear Accumulation of Heat-shock Protein 90 Is Associated with Poor Survival and Metastasis in Patients with Non-small Cell Lung Cancer. Anticancer Res. 2016, 36, 2197–2203. [Google Scholar] [PubMed]
- Lee, J.H.; Chung, I.K. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010, 290, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Tapia, H.; Morano, K.A. Hsp90 Nuclear Accumulation in Quiescence Is Linked to Chaperone Function and Spore Development in Yeast. Mol. Biol. Cell 2010, 21, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Toogun, O.A.; DeZwaan, D.C.; Freeman, B.C. The Hsp90 Molecular Chaperone Modulates Multiple Telomerase Activities. Mol. Cell. Biol. 2008, 28, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Echeverría, P.C.; Erlejman, A.G.; Piwien-Pilipuk, G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 2010, 1, 299–308. [Google Scholar] [CrossRef]
- Kim, H.L.; Cassone, M.; Otvos, L., Jr.; Vogiatzi, P. HIF-1α and STAT3 client proteins interacting with the cancer chaperone Hsp90: Therapeutic considerations. Cancer Biol. Ther. 2008, 7, 10–14. [Google Scholar] [CrossRef]
- Echeverria, P.C.; Mazaira, G.; Erlejman, A.; Gomez-Sanchez, C.; Piwien Pilipuk, G.; Galigniana, M.D. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol. Cell Biol. 2009, 29, 4788–4797. [Google Scholar] [CrossRef]
- Langer, T.; Rosmus, S.; Fasold, H. Intracellular localization of the 90 kDA heat shock protein (HSP90α) determined by expression of a EGFP-HSP90α-fusion protein in unstressed and heat stressed 3T3 cells. Cell Biol. Int. 2003, 27, 47–52. [Google Scholar] [CrossRef]
- Garg, G.; Khandelwal, A.; Blagg, B.S. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv. Cancer Res. 2016, 129, 51–88. [Google Scholar]
- Moser, C.; Lang, S.A.; Stoeltzing, O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009, 29, 2031–2042. [Google Scholar]
- Kang, B.H.; Altieri, D.C. Compartmentalized cancer drug discovery targeting mitochondrial Hsp90 chaperones. Oncogene 2009, 28, 3681–3688. [Google Scholar] [CrossRef]
- Altieri, D.C. Mitochondrial Hsp90 chaperones as novel molecular targets in prostate cancer. Futur. Oncol. 2010, 6, 487–489. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.H.; Tavecchio, M.; Goel, H.L.; Hsieh, C.-C.; Garlick, D.S.; Raskett, C.M.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease. Br. J. Cancer 2011, 104, 629–634. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Dohi, T.; Raskett, C.M.; Orlowski, G.M.; Powers, C.M.; Gilbert, C.A.; Ross, A.H.; Plescia, J.; Altieri, D.C. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J. Clin. Investig. 2011, 121, 1349–1360. [Google Scholar] [CrossRef]
- Siegelin, M.D. Inhibition of the mitochondrial Hsp90 chaperone network: A novel, efficient treatment strategy for cancer? Cancer Lett. 2013, 333, 133–146. [Google Scholar] [CrossRef]
- Seo, Y.H. Organelle-specific Hsp90 inhibitors. Arch. Pharmacal Res. 2015, 38, 1582–1590. [Google Scholar] [CrossRef]
- Bryant, K.G.; Chae, Y.C.; Martinez, R.L.; Gordon, J.C.; Elokeley, K.M.; Kossenkov, A.V.; Grant, S.; Childers, W.E.; Abou-Gharbia, M.; Altieri, D.C. A Mitochondrial-targeted purine-based HSP90 antagonist for leukemia therapy. Oncotarget 2017, 8, 112184–112198. [Google Scholar] [CrossRef]
- Zhang, G.; Frederick, D.T.; Wu, L.; Wei, Z.; Krepler, C.; Srinivasan, S.; Chae, Y.C.; Xu, X.; Choi, H.; Dimwamwa, E.; et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J. Clin. Investig. 2016, 126, 1834–1856. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.C.; Angelin, A.; Lisanti, S.; Kossenkov, A.V.; Speicher, K.D.; Wang, H.; Powers, J.; Tischler, A.; Pacak, K.; Fliedner, S.; et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat. Commun. 2013, 4, 2139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Elnatan, D.; Sun, M.; Myasnikov, A.G.; Agard, D.A. Cryo-EM reveals the dynamic interplay between mitochondrial Hsp90 and SdhB folding intermediates. BioRxiv 2020. [Google Scholar] [CrossRef]
- Schulte, T.W.; Blagosklonny, M.V.; Romanova, L.; Mushinski, J.F.; Monia, B.P.; Johnston, J.F.; Nguyen, P.; Trepel, J.; Neckers, L.M. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol. Cell. Biol. 1996, 16, 5839–5845. [Google Scholar] [CrossRef]
- Stepanova, L.; Leng, X.; Parker, S.B.; Harper, J.W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996, 10, 1491–1502. [Google Scholar] [CrossRef] [Green Version]
- Shiotsu, Y.; Soga, S.; Akinaga, S. Heat Shock Protein 90-antagonist Destabilizes Bcr-Abl/HSP90 Chaperone Complex. Leuk. Lymphoma 2002, 43, 961–968. [Google Scholar] [CrossRef]
- Xu, Y.; Lindquist, S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc. Natl. Acad. Sci. USA 1993, 90, 7074–7078. [Google Scholar] [CrossRef]
- Xu, Y.; Singer, M.A.; Lindquist, S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA 1999, 96, 109–114. [Google Scholar] [CrossRef]
- Martins, A.S.; Davies, F.; Workman, P. Inhibiting the molecular evolution of cancer through HSP90. Oncotarget 2012, 3, 1054–1056. [Google Scholar] [CrossRef]
- Miyata, Y.; Yahara, I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 1992, 267, 7042–7047. [Google Scholar] [CrossRef]
- Arlander, S.J.; Eapen, A.K.; Vroman, B.T.; McDonald, R.J.; Toft, D.O.; Karnitz, L.M. Hsp90 Inhibition Depletes Chk1 and Sensitizes Tumor Cells to Replication Stress. J. Biol. Chem. 2003, 278, 52572–52577. [Google Scholar] [CrossRef]
- Uma, S.; Hartson, S.D.; Chen, J.-J.; Matts, R.L. Hsp90 Is Obligatory for the Heme-regulated eIF-2α Kinase to Acquire and Maintain an Activable Conformation. J. Biol. Chem. 1997, 272, 11648–11656. [Google Scholar] [CrossRef]
- Fortugno, P.; Beltrami, E.; Plescia, J.; Fontana, J.; Pradhan, D.; Marchisio, P.C.; Sessa, W.C.; Altieri, D.C. Regulation of survivin function by Hsp90. Proc. Natl. Acad. Sci. USA 2003, 100, 13791–13796. [Google Scholar] [CrossRef]
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol. Cell Biol. 2008, 28, 3344–3358. [Google Scholar] [CrossRef]
- Broemer, M.; Krappmann, D.; Scheidereit, C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene 2004, 23, 5378–5386. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, J.; Farah, E.; Sarkar, S.; Ahmad, N.; Gupta, S.; Larner, J.; Liu, X. Cotargeting HSP90 and Its Client Proteins for Treatment of Prostate Cancer. Mol. Cancer Ther. 2016, 15, 2107–2118. [Google Scholar] [CrossRef]
- Aligue, R.; Akhavan-Niak, H.; Russell, P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994, 13, 6099–6106. [Google Scholar] [CrossRef]
- Tang, X.X.; Regan, P.L.; Jacobs, J.; Wang, G.; Torres, J.; Edo, R.; Friedmann, J. Tang Hsp90 inhibition increases p53 expression and destabilizes MYCN and MYC in neuroblastoma. Int. J. Oncol. 2010, 38, 105–112. [Google Scholar] [CrossRef]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef]
- Smith-Jones, P.M.; Solit, D.B.; Akhurst, T.; Afroze, F.; Rosen, N.; Larson, S.M. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat. Biotechnol. 2004, 22, 701–706. [Google Scholar] [CrossRef]
- Han, S.-Y. Small Molecule Induced FLT3 Degradation. Pharmaceuticals 2022, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Masson-Gadais, B.; Houle, F.; Laferriere, J.; Huot, J. Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress Chaperones. 2003, 8, 37–52. [Google Scholar] [CrossRef]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt Forms an Intracellular Complex with Heat Shock Protein 90 (Hsp90) and Cdc37 and Is Destabilized by Inhibitors of Hsp90 Function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pashtan, I.; Tsutsumi, S.; Xu, W.; Neckers, L. Cancer cells harboring MET gene amplification activate alternative signaling pathways to escape MET inhibition but remain sensitive to Hsp90 inhibitors. Cell Cycle 2009, 8, 2050–2056. [Google Scholar] [CrossRef]
- Eustace, B.K.; Sakurai, T.; Stewart, J.K.; Yimlamai, D.; Unger, C.; Zehetmeier, C.; Lain, B.; Torella, C.; Henning, S.W.; Beste, G.; et al. Functional proteomic screens reveal an essential extracellular role for hsp90α in cancer cell invasiveness. Nat. Cell Biol. 2004, 6, 507–514. [Google Scholar] [CrossRef]
- Zhang, P.C.; Liu, X.; Li, M.M.; Ma, Y.Y.; Sun, H.T.; Tian, X.Y.; Wang, Y.; Liu, M.; Fu, L.S.; Wang, Y.F.; et al. AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1alpha/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo. Biochem. Pharmacol. 2020, 172, 113771. [Google Scholar] [CrossRef]
- Howard, K.J.; Holley, S.J.; Yamamoto, K.R.; Distelhorst, C.W. Mapping the HSP90 binding region of the glucocorticoid receptor. J. Biol. Chem. 1990, 265, 11928–11935. [Google Scholar] [CrossRef]
- Kovacs, J.J.; Murphy, P.J.M.; Gaillard, S.; Zhao, X.; Wu, J.-T.; Nicchitta, C.V.; Yoshida, M.; Toft, D.O.; Pratt, W.B.; Yao, T.-P. HDAC6 Regulates Hsp90 Acetylation and Chaperone-Dependent Activation of Glucocorticoid Receptor. Mol. Cell 2005, 18, 601–607. [Google Scholar] [CrossRef]
- Smith, D.F.; Whitesell, L.; Nair, S.C.; Chen, S.; Prapapanich, V.; Rimerman, A.R. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol. Cell. Biol. 1995, 15, 6804–6812. [Google Scholar] [CrossRef]
- Sabbah, M.; Radanyi, C.; Redeuilh, G.; Baulieu, E.-E. The 90 kDa heat-shock protein (hsp90) modulates the binding of the oestrogen receptor to its cognate DNA. Biochem. J. 1996, 314, 205–213. [Google Scholar] [CrossRef]
- Knoblauch, R.; Garabedian, M.J. Role for Hsp90-Associated Cochaperone p23 in Estrogen Receptor Signal Transduction. Mol. Cell. Biol. 1999, 19, 3748–3759. [Google Scholar] [CrossRef]
- Fliss, A.E.; Benzeno, S.; Rao, J.; Caplan, A.J. Control of estrogen receptor ligand binding by Hsp90. J. Steroid Biochem. Mol. Biol. 2000, 72, 223–230. [Google Scholar] [CrossRef]
- Ni, L.; Yang, C.-S.; Gioeli, D.; Frierson, H.; Toft, D.O.; Paschal, B.M. FKBP51 Promotes Assembly of the Hsp90 Chaperone Complex and Regulates Androgen Receptor Signaling in Prostate Cancer Cells. Mol. Cell. Biol. 2010, 30, 1243–1253. [Google Scholar] [CrossRef]
- Loo, M.A.; Jensen, T.J.; Cui, L.; Hou, Y.-X.; Chang, X.-B.; Riordan, J.R. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998, 17, 6879–6887. [Google Scholar] [CrossRef]
- García-Cardeña, G.; Fan, R.; Shah, V.; Sorrentino, R.; Cirino, G.; Papapetropoulos, A.; Sessa, W.C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 1998, 392, 821–824. [Google Scholar] [CrossRef]
- Ding, G.; Chen, P.; Zhang, H.; Huang, X.; Zang, Y.; Li, J.; Li, J.; Wong, J. Regulation of Ubiquitin-like with Plant Homeodomain and RING Finger Domain 1 (UHRF1) Protein Stability by Heat Shock Protein 90 Chaperone Machinery. J. Biol. Chem. 2016, 291, 20125–20135. [Google Scholar] [CrossRef]
- Noguchi, M.; Yu, D.; Hirayama, R.; Ninomiya, Y.; Sekine, E.; Kubota, N.; Ando, K.; Okayasu, R. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem. Biophys. Res. Commun. 2006, 351, 658–663. [Google Scholar] [CrossRef]
- Bradley, E.; Bieberich, E.; Mivechi, N.F.; Tangpisuthipongsa, D.; Wang, G. Regulation of Embryonic Stem Cell Pluripotency by Heat Shock Protein 90. Stem Cells 2012, 30, 1624–1633. [Google Scholar] [CrossRef]
- Roby, J.A.; Esser-Nobis, K.; Dewey-Verstelle, E.C.; Fairgrieve, M.R.; Schwerk, J.; Lu, A.Y.; Soveg, F.W.; Hemann, E.A.; Hatfield, L.D.; Keller, B.C.; et al. Flavivirus Nonstructural Protein NS5 Dysregulates HSP90 to Broadly Inhibit JAK/STAT Signaling. Cells 2020, 9, 899. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; He, L.; Xiang, Y.; Tian, C.; Li, C.; Tan, P.; Jing, J.; Tian, Y.; Du, L.; et al. Discovery of Small-Molecule Inhibitors of the HSP90-Calcineurin-NFAT Pathway against Glioblastoma. Cell Chem. Biol. 2019, 26, 352–365.e7. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Hiltunen, M.; Soininen, H. Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer’s disease. Prog. Neurobiol. 2011, 93, 99–110. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K. The HSP90 complex of plants. Biochim. Biophys. Acta Bioenergy 2012, 1823, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, L.; Li, C.; Lu, W.; Chen, J. Inhibition of MDM2 by hsp90 Contributes to Mutant p53 Stabilization. J. Biol. Chem. 2001, 276, 40583–40590. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Marchenko, N.D.; Schulz, R.; Fischer, V.; Velasco-Hernandez, T.; Talos, F.; Moll, U.M. Functional Inactivation of Endogenous MDM2 and CHIP by HSP90 Causes Aberrant Stabilization of Mutant p53 in Human Cancer Cells. Mol. Cancer Res. 2011, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C.; Stein, G.S.; Lian, J.B.; Languino, L.R. TRAP-1, the mitochondrial Hsp90. Biochim. Biophys. Acta 2012, 1823, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Park, H.-K.; Jeong, H.; Lim, J.; Lee, A.-J.; Cheon, K.Y.; Kim, C.-S.; Thomas, A.P.; Bae, B.; Kim, N.D.; et al. Development of a Mitochondria-Targeted Hsp90 Inhibitor Based on the Crystal Structures of Human TRAP1. J. Am. Chem. Soc. 2015, 137, 4358–4367. [Google Scholar] [CrossRef]
- Sung, N.; Lee, J.; Kim, J.-H.; Chang, C.; Joachimiak, A.; Lee, S.; Tsai, F.T.F. Mitochondrial Hsp90 is a ligand-activated molecular chaperone coupling ATP binding to dimer closure through a coiled-coil intermediate. Proc. Natl. Acad. Sci. USA 2016, 113, 2952–2957. [Google Scholar] [CrossRef]
- Condelli, V.; Crispo, F.; Pietrafesa, M.; Lettini, G.; Matassa, D.S.; Esposito, F.; Landriscina, M.; Maddalena, F. HSP90 Molecular Chaperones, Metabolic Rewiring, and Epigenetics: Impact on Tumor Progression and Perspective for Anticancer Therapy. Cells 2019, 8, 532. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Scroggins, B.; Koga, F.; Lee, M.J.; Trepel, J.; Felts, S.; Carreras, C.; Neckers, L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2008, 27, 2478–2487. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef]
- Barrott, J.J.; Haystead, T.A.J. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.J.; Robinson, A.E.; Law, L.W.; Willingham, M.; Appella, E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc. Natl. Acad. Sci. USA 1986, 83, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.-F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef]
- Yang, Y.; Rao, R.; Shen, J.; Tang, Y.; Fiskus, W.; Nechtman, J.; Atadja, P.; Bhalla, K. Role of Acetylation and Extracellular Location of Heat Shock Protein 90α in Tumor Cell Invasion. Cancer Res. 2008, 68, 4833–4842. [Google Scholar] [CrossRef] [PubMed]
- Eustace, B.K.; Jay, D.G. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle 2004, 3, 1096–1098. [Google Scholar] [CrossRef] [Green Version]
- Stellas, D.; El Hamidieh, A.; Patsavoudi, E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Gaitanou, M.; Stellas, D.; Matsas, R.; Patsavoudi, E. A Critical Role for HSP90 in Cancer Cell Invasion Involves Interaction with the Extracellular Domain of HER-2. J. Biol. Chem. 2008, 283, 2031–2041. [Google Scholar] [CrossRef]
- Woodley, D.T.; Fan, J.; Cheng, C.-F.; Li, Y.; Chen, M.; Bu, G.; Li, W. Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90α autocrine signaling to promote keratinocyte migration. J. Cell Sci. 2009, 122, 1495–1498. [Google Scholar] [CrossRef]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 Is a Novel Regulator of the Epithelial to Mesenchymal Transition (EMT) in Prostate Cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef]
- Bishop, S.; Burlison, J.; Blagg, B.J. Hsp90: A Novel Target for the Disruption of Multiple Signaling Cascades. Curr. Cancer Drug Targets 2007, 7, 369–388. [Google Scholar] [CrossRef]
- Smith, J.R.; Workman, P. Targeting the cancer chaperone HSP90. Drug Discov. Today. Ther. Strateg. 2007, 4, 219–227. [Google Scholar] [CrossRef]
- Theodoraki, M.A.; Caplan, A.J. Quality control and fate determination of Hsp90 client proteins. Biochim. Biophys. Acta 2012, 1823, 683–688. [Google Scholar] [CrossRef]
- Xu, W.; Mimnaugh, E.G.; Kim, J.S.; Trepel, J.B.; Neckers, L.M. Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones. 2002, 7, 91–96. [Google Scholar] [CrossRef]
- Blagosklonny, M.; Fojo, T.; Bhalla, K.; Kim, J.-S.; Trepel, J.; Figg, W.; Rivera, Y.; Neckers, L. The Hsp90 inhibitor geldanamycin selectively sensitizes Bcr-Abl-expressing leukemia cells to cytotoxic chemotherapy. Leukemia 2001, 15, 1537–1543. [Google Scholar] [CrossRef]
- Pinhasi-Kimhi, O.; Michalovitz, D.; Ben-Zeev, A.; Oren, M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature 1986, 320, 182–185. [Google Scholar] [CrossRef]
- Wiech, M.; Olszewski, M.B.; Tracz-Gaszewska, Z.; Wawrzynow, B.; Zylicz, M.; Zylicz, A. Molecular Mechanism of Mutant p53 Stabilization: The Role of HSP70 and MDM2. PLoS ONE 2012, 7, e51426. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Toretsky, J.; Bohen, S.; Neckers, L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc. Natl. Acad. Sci. USA 1996, 93, 8379–8383. [Google Scholar] [CrossRef]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.; Liu, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef]
- Dias, S.; Shmelkov, S.V.; Lam, G.; Rafii, S. VEGF165 promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 2002, 99, 2532–2540. [Google Scholar] [CrossRef]
- Forsythe, H.L.; Jarvis, J.L.; Turner, J.W.; Elmore, L.W.; Holt, S.E. Stable Association of hsp90 and p23, but Not hsp70, with Active Human Telomerase. J. Biol. Chem. 2001, 276, 15571–15574. [Google Scholar] [CrossRef]
- Walsh, N.; Larkin, A.; Swan, N.; Conlon, K.; Dowling, P.; McDermott, R.; Clynes, M. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Lett. 2011, 306, 180–189. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Long, T.-E.; Park, W.; Landry, J.C.; Taliaferro-Smith, L.; Farris, A.; Diaz, R.; El-Rayes, B.F. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol. Carcinog. 2014, 54, 1147–1158. [Google Scholar] [CrossRef]
- Miyajima, N.; Tsutsumi, S.; Sourbier, C.; Beebe, K.; Mollapour, M.; Rivas, C.; Yoshida, S.; Trepel, J.B.; Huang, Y.; Tatokoro, M.; et al. The HSP90 Inhibitor Ganetespib Synergizes with the MET Kinase Inhibitor Crizotinib in both Crizotinib-Sensitive and -Resistant MET-Driven Tumor Models. Cancer Res. 2013, 73, 7022–7033. [Google Scholar] [CrossRef]
- Isaacs, J.S.; Jung, Y.-J.; Mimnaugh, E.G.; Martinez, A.; Cuttitta, F.; Neckers, L.M. Hsp90 Regulates a von Hippel Lindau-independent Hypoxia-inducible Factor-1α-degradative Pathway. J. Biol. Chem. 2002, 277, 29936–29944. [Google Scholar] [CrossRef]
- Whittier, J.E.; Xiong, Y.; Rechsteiner, M.C.; Squier, T.C. Hsp90 Enhances Degradation of Oxidized Calmodulin by the 20 S Proteasome. J. Biol. Chem. 2004, 279, 46135–46142. [Google Scholar] [CrossRef] [Green Version]
- Stebbins, E.C.; Russo, A.A.; Schneider, C.; Rosen, N.; Hartl, F.; Pavletich, N.P. Crystal Structure of an Hsp90-Geldanamycin Complex: Targeting of a Protein Chaperone by an Antitumor Agent. Cell 1997, 89, 239–250. [Google Scholar] [CrossRef]
- Wu, M.-S.; Lien, G.-S.; Shen, S.-C.; Yang, L.-Y.; Chen, Y.-C. HSP90 Inhibitors, Geldanamycin and Radicicol, Enhance Fisetin-Induced Cytotoxicity via Induction of Apoptosis in Human Colonic Cancer Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 987612. [Google Scholar] [CrossRef]
- Schulte, T.W.; Neckers, L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998, 42, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gürpınar, T.; Kosova, F.; Kurt, F.O.; Cambaz, S.U.; Yücel, A.T.; Umur, N.; Tuğlu, M.I. Effect of geldanamycin on the expression of the matrix molecules and angiogenetic factors in a gastric cancer cell line. Biotech. Histochem. 2020, 96, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Belotti, D.; Burger, A.M.; Fisher-Nielson, K.; Borsotti, P.; Riccardi, E.; Thillainathan, J.; Hollingshead, M.; Sausville, E.A.; Giavazzi, R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: An orally bioavailable heat shock protein 90 modulator. Clin. Cancer Res. 2004, 10, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Schwock, J.; Pham, N.-A.; Cao, M.P.; Hedley, D.W.; Schwock, J. Efficacy of Hsp90 inhibition for induction of apoptosis and inhibition of growth in cervical carcinoma cells in vitro and in vivo. Cancer Chemother. Pharmacol. 2007, 61, 669–681. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, F. Effects of 17-DMAG on non-small cell lung cancer cell lines A549 and H1975 being resistant to EGFR-TK. Zhongguo Fei Ai Za Zhi. 2014, 17, 778–782. [Google Scholar]
- Patterson, J.; Palombella, V.J.; Fritz, C.; Normant, E. IPI-504, a novel and soluble HSP-90 inhibitor, blocks the unfolded protein response in multiple myeloma cells. Cancer Chemother. Pharmacol. 2007, 61, 923–932. [Google Scholar] [CrossRef]
- Stingl, L.; Stühmer, T.; Chatterjee, M.; Jensen, M.R.; Flentje, M.; Djuzenova, C.S. Novel HSP90 inhibitors, NVP-AUY922 and NVP-BEP800, radiosensitise tumour cells through cell-cycle impairment, increased DNA damage and repair protraction. Br. J. Cancer 2010, 102, 1578–1591. [Google Scholar] [CrossRef]
- Stühmer, T.; Chatterjee, M.; Grella, E.; Seggewiss, R.; Langer, C.; Müller, S.; Schoepfer, J.; Garcia-Echeverria, C.; Quadt, C.; Jensen, M.R.; et al. Anti-myeloma activity of the novel 2-aminothienopyrimidine Hsp90 inhibitor NVP-BEP800. Br. J. Haematol. 2009, 147, 319–327. [Google Scholar] [CrossRef]
- Ohkubo, S.; Kodama, Y.; Muraoka, H.; Hitotsumachi, H.; Yoshimura, C.; Kitade, M.; Hashimoto, A.; Ito, K.; Gomori, A.; Takahashi, K.; et al. TAS-116, a Highly Selective Inhibitor of Heat Shock Protein 90α and β, Demonstrates Potent Antitumor Activity and Minimal Ocular Toxicity in Preclinical Models. Mol. Cancer Ther. 2015, 14, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Kurokawa, Y.; Sawaki, A.; Komatsu, Y.; Ozaka, M.; Takahashi, T.; Naito, Y.; Ohkubo, S.; Nishida, T. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: A phase II, single-arm trial. Eur. J. Cancer 2019, 121, 29–39. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Song, H.Y.; Meli, M.; Colombo, G.; Beebe, K.; Scroggins, B.; Neckers, L.; Altieri, D.C. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J. Clin. Investig. 2009, 119, 454–464. [Google Scholar] [CrossRef]
- Wei, S.; Yin, D.; Yu, S.; Lin, X.; Savani, M.R.; Du, K.; Ku, Y.; Wu, D.; Li, S.; Liu, H.; et al. Antitumor Activity of a Mitochondrial-Targeted HSP90 Inhibitor in Gliomas. Clin. Cancer Res. 2022, 28, 2180–2195. [Google Scholar] [CrossRef]
- Chandarlapaty, S.; Sawai, A.; Ye, Q.; Scott, A.; Silinski, M.; Huang, K.; Fadden, P.; Partdrige, J.; Hall, S.; Steed, P.; et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin. Cancer Res. 2008, 14, 240–248. [Google Scholar] [CrossRef]
- Martin, C.J.; Gaisser, S.; Challis, I.R.; Carletti, I.; Wilkinson, B.; Gregory, M.; Prodromou, C.; Roe, S.M.; Pearl, L.H.; Boyd, S.M.; et al. Molecular Characterization of Macbecin as an Hsp90 Inhibitor. J. Med. Chem. 2008, 51, 2853–2857. [Google Scholar] [CrossRef] [PubMed]
- Bussenius, J.; Blazey, C.M.; Aay, N.; Anand, N.K.; Arcalas, A.; Baik, T.; Bowles, O.J.; Buhr, C.A.; Costanzo, S.; Curtis, J.K.; et al. Discovery of XL888: A novel tropane-derived small molecule inhibitor of HSP90. Bioorganic Med. Chem. Lett. 2012, 22, 5396–5404. [Google Scholar] [CrossRef] [PubMed]
- Haarberg, H.E.; Paraiso, K.H.; Wood, E.; Rebecca, V.W.; Sondak, V.K.; Koomen, J.M.; Smalley, K.S. Inhibition of Wee1, AKT, and CDK4 underlies the efficacy of the HSP90 inhibitor XL888 in an in vivo model of NRAS-mutant melanoma. Mol. Cancer Ther. 2013, 12, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Bai, M.; Ke, W.; Wang, X.; Zhao, X.; Lu, Z. The HSP90 inhibitor, XL888, enhanced cell apoptosis via downregulating STAT3 after insufficient radiofrequency ablation in hepatocellular carcinoma. Life Sci. 2021, 282, 119762. [Google Scholar] [CrossRef]
- Akimoto, T.; Nonaka, T.; Harashima, K.; Sakurai, H.; Ishikawa, H.; Mitsuhashi, N. Radicicol potentiates heat-induced cell killing in a human oesophageal cancer cell line: The Hsp90 chaperone complex as a new molecular target for enhancement of thermosensitivity. Int. J. Radiat. Biol. 2004, 80, 483–492. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.A.; Myung, S.C.; Kim, W.; Lee, C.S. Radicicol, an inhibitor of Hsp90, enhances TRAIL-induced apoptosis in human epithelial ovarian carcinoma cells by promoting activation of apoptosis-related proteins. Mol. Cell. Biochem. 2011, 359, 33–43. [Google Scholar] [CrossRef]
- Wang, Y.; Trepel, J.B.; Neckers, L.M.; Giaccone, G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr. Opin. Investig. Drugs 2010, 11, 1466–1476. [Google Scholar]
- Shah, S.; Luke, J.J.; Jacene, H.A.; Chen, T.; Giobbie-Hurder, A.; Ibrahim, N.; Buchbinder, E.L.; McDermott, D.F.; Flaherty, K.T.; Sullivan, R.J.; et al. Results from phase II trial of HSP90 inhibitor, STA-9090 (ganetespib), in metastatic uveal melanoma. Melanoma Res. 2018, 28, 605–610. [Google Scholar] [CrossRef]
- Smith, N.F.; Hayes, A.; James, K.; Nutley, B.P.; McDonald, E.; Henley, A.; Dymock, B.; Drysdale, M.J.; Raynaud, F.I.; Workman, P. Preclinical pharmacokinetics and metabolism of a novel diaryl pyrazole resorcinol series of heat shock protein 90 inhibitors. Mol. Cancer Ther. 2006, 5, 1628–1637. [Google Scholar] [CrossRef]
- Sharp, S.Y.; Boxall, K.; Rowlands, M.; Prodromou, C.; Roe, S.M.; Maloney, A.; Powers, M.; Clarke, P.A.; Box, G.; Sanderson, S.; et al. In vitro Biological Characterization of a Novel, Synthetic Diaryl Pyrazole Resorcinol Class of Heat Shock Protein 90 Inhibitors. Cancer Res. 2007, 67, 2206–2216. [Google Scholar] [CrossRef]
- Jeong, J.H.; Oh, Y.J.; Kwon, T.K.; Seo, Y.H. Chalcone-templated Hsp90 inhibitors and their effects on gefitinib resistance in non-small cell lung cancer (NSCLC). Arch. Pharmacal Res. 2016, 40, 96–105. [Google Scholar] [CrossRef]
- Yong, K.; Cavet, J.; Johnson, P.; Morgan, G.; Williams, C.; Nakashima, D.; Akinaga, S.; Oakervee, H.; Cavenagh, J. Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies. Br. J. Cancer. 2016, 114, 7–13. [Google Scholar] [CrossRef]
- Woodhead, A.J.; Angove, H.; Carr, M.G.; Chessari, G.; Congreve, M.; Coyle, J.E.; Cosme, J.; Graham, B.; Day, P.J.; Downham, R.; et al. Discovery of (2,4-Dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1,3-dihydroisoindol-2-yl]methanone (AT13387), a Novel Inhibitor of the Molecular Chaperone Hsp90 by Fragment Based Drug Design. J. Med. Chem. 2010, 53, 5956–5969. [Google Scholar] [CrossRef]
- Graham, B.; Curry, J.; Smyth, T.; Fazal, L.; Feltell, R.; Harada, I.; Coyle, J.; Williams, B.; Reule, M.; Angove, H.; et al. The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci. 2012, 103, 522–527. [Google Scholar] [CrossRef]
- Hadden, M.K.; Lubbers, D.J.; Blagg, B.S. Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N-terminal ATP binding site. Curr. Top. Med. Chem. 2006, 6, 1173–1182. [Google Scholar] [CrossRef]
- Taldone, T.; Chiosis, G. Purine-scaffold Hsp90 inhibitors. Curr. Top. Med. Chem. 2009, 9, 1436–1446. [Google Scholar] [CrossRef]
- Lundgren, K.; Zhang, H.; Brekken, J.; Huser, N.; Powell, R.E.; Timple, N.; Busch, D.J.; Neely, L.; Sensintaffar, J.L.; Yang, Y.-C.; et al. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol. Cancer Ther. 2009, 8, 921–929. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Hu, H. BIIB021, an Hsp90 inhibitor: A promising therapeutic strategy for blood malignancies (Review). Oncol. Rep. 2018. [Google Scholar] [CrossRef]
- Hong, D.; Said, R.; Falchook, G.; Naing, A.; Moulder, S.; Tsimberidou, A.-M.; Galluppi, G.; Dakappagari, N.; Storgard, C.; Kurzrock, R.; et al. Phase I Study of BIIB028, a Selective Heat Shock Protein 90 Inhibitor, in Patients with Refractory Metastatic or Locally Advanced Solid Tumors. Clin. Cancer Res. 2013, 19, 4824–4831. [Google Scholar] [CrossRef]
- Park, H.-K.; Yoon, N.G.; Lee, J.-E.; Hu, S.; Yoon, S.; Kim, S.Y.; Hong, J.-H.; Nam, D.; Chae, Y.C.; Park, J.B.; et al. Unleashing the full potential of Hsp90 inhibitors as cancer therapeutics through simultaneous inactivation of Hsp90, Grp94, and TRAP1. Exp. Mol. Med. 2020, 52, 79–91. [Google Scholar] [CrossRef]
- Kim, S.-H.; Tangallapally, R.; Kim, I.C.; Trovato, R.; Parker, D.P.; Patton, J.S.; Reeves, L.; Bradford, C.; Wettstein, D.; Baichwal, V.; et al. Discovery of an l-alanine ester prodrug of the Hsp90 inhibitor, MPC-3100. Bioorganic Med. Chem. Lett. 2015, 25, 5254–5257. [Google Scholar] [CrossRef]
- Eder, J.P.; Wheeler, A.C.; Teicher, A.B.; Schnipper, L.E. A phase I clinical trial of novobiocin, a modulator of alkylating agent cytotoxicity. Cancer Res. 1991, 51, 510–513. [Google Scholar]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Romano, A.; Martel, F. The Role of EGCG in Breast Cancer Prevention and Therapy. Mini Rev. Med. Chem. 2021, 21, 883–898. [Google Scholar] [CrossRef]
- Gazák, R.; Walterova, D.; Kren, V. Silybin and Silymarin—New and Emerging Applications in Medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Galanski, M. Recent developments in the field of anticancer platinum complexes. Recent Pat. Anti-Cancer Drug Discov. 2006, 1, 285–295. [Google Scholar] [CrossRef]
- Hohnloser, J.H.; Schierl, R.; Hasford, B.; Emmerich, B. Cisplatin based chemotherapy in testicular cancer patients: Long term platinum excretion and clinical effects. Eur. J. Med. Res. 1996, 1, 509–514. [Google Scholar]
- Blanářová, O.V.; Šafaříková, B.; Herůdková, J.; Krkoška, M.; Tománková, S.; Kahounová, Z.; Anděra, L.; Bouchal, J.; Kharaishvili, G.; Král, M.; et al. Cisplatin or LA-12 enhance killing effects of TRAIL in prostate cancer cells through Bid-dependent stimulation of mitochondrial apoptotic pathway but not caspase-10. PLoS ONE 2017, 12, e0188584. [Google Scholar] [CrossRef]
- Kvardova, V.; Hrstka, R.; Walerych, D.; Muller, P.; Matoulkova, E.; Hruskova, V.; Stelclova, D.; Sova, P.; Vojtesek, B. The new platinum(IV) derivative LA-12 shows stronger inhibitory effect on Hsp90 function compared to cisplatin. Mol. Cancer 2010, 9, 147. [Google Scholar] [CrossRef] [Green Version]
- Žák, F.; Turánek, J.; Kroutil, A.; Sova, P.; Mistr, A.; Poulová, A.; Mikolin, P.; Žák, Z.; Kašná, A.; Záluská, D.; et al. Platinum(IV) Complex with Adamantylamine as Nonleaving Amine Group: Synthesis, Characterization, and in Vitro Antitumor Activity against a Panel of Cisplatin-Resistant Cancer Cell Lines. J. Med. Chem. 2003, 47, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.A.; Bornmann, W.; Erdjument-Bromage, H.; Tempst, P.; Pavletich, N.; Rosen, N.; Nathan, C.F.; Ding, A. Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. USA 1999, 96, 5645–5650. [Google Scholar] [CrossRef] [PubMed]
- Vasko, R.C.; Rodriguez, R.A.; Cunningham, C.N.; Ardi, V.C.; Agard, D.A.; McAlpine, S.R. Mechanistic Studies of Sansalvamide A-Amide: An Allosteric Modulator of Hsp90. ACS Med. Chem. Lett. 2010, 1, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Heiferman, M.J.; Salabat, M.R.; Ujiki, M.B.; Strouch, M.J.; Cheon, E.C.; Silverman, R.B.; Bentrem, D.J. Sansalvamide induces pancreatic cancer growth arrest through changes in the cell cycle. Anticancer Res. 2010, 30, 73–78. [Google Scholar] [PubMed]
- Lee, S.-C.; Min, H.-Y.; Choi, H.; Kim, H.S.; Kim, K.-C.; Park, S.-J.; Seong, A.M.; Seo, J.H.; Park, H.-J.; Suh, Y.-G.; et al. Synthesis and Evaluation of a Novel Deguelin Derivative, L80, which Disrupts ATP Binding to the C-terminal Domain of Heat Shock Protein 90. Mol. Pharmacol. 2015, 88, 245–255. [Google Scholar] [CrossRef]
- Plescia, J.; Salz, W.; Xia, F.; Pennati, M.; Zaffaroni, N.; Daidone, M.G.; Meli, M.; Dohi, T.; Fortugno, P.; Nefedova, Y.; et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell 2005, 7, 457–468. [Google Scholar] [CrossRef]
- Rajan, A.; Kelly, R.J.; Trepel, J.B.; Kim, Y.S.; Alarcon, S.V.; Kummar, S.; Gutierrez, M.; Crandon, S.; Zein, W.M.; Jain, L.; et al. A Phase I Study of PF-04929113 (SNX-5422), an Orally Bioavailable Heat Shock Protein 90 Inhibitor, in Patients with Refractory Solid Tumor Malignancies and Lymphomas. Clin. Cancer Res. 2011, 17, 6831–6839. [Google Scholar] [CrossRef]
- Reddy, N.; Voorhees, P.M.; Houk, B.E.; Brega, N.; Hinson, J.M.; Jillela, A. Phase I Trial of the HSP90 Inhibitor PF-04929113 (SNX5422) in Adult Patients with Recurrent, Refractory Hematologic Malignancies. Clin. Lymphoma Myeloma Leuk. 2013, 13, 385–391. [Google Scholar] [CrossRef]
- Stühmer, T.; Iskandarov, K.; Gao, Z.; Bumm, T.; Grella, E.; Jensen, M.R.; Einsele, H.; Chatterjee, M.; Bargou, R.C. Preclinical activity of the novel orally bioavailable HSP90 inhibitor NVP-HSP990 against multiple myeloma cells. Anticancer Res. 2012, 32, 453–462. [Google Scholar]
- Liu, J.; Wu, X.-D.; Li, W.; Yuan, Z.; Yang, K.; Zhao, Q.-S. Discovery of pseudolaric acid A as a new Hsp90 inhibitor uncovers its potential anticancer mechanism. Bioorganic Chem. 2021, 112, 104963. [Google Scholar] [CrossRef]
- Suda, A.; Kawasaki, K.-I.; Komiyama, S.; Isshiki, Y.; Yoon, D.-O.; Kim, S.-J.; Na, Y.-J.; Hasegawa, K.; Fukami, T.A.; Sato, S.; et al. Design and synthesis of 2-amino-6-(1H,3H-benzo[de]isochromen-6-yl)-1,3,5-triazines as novel Hsp90 inhibitors. Bioorganic Med. Chem. 2013, 22, 892–905. [Google Scholar] [CrossRef] [Green Version]
- Fogliatto, G.; Gianellini, L.; Brasca, M.G.; Casale, E.; Ballinari, D.; Ciomei, M.; Degrassi, A.; De Ponti, A.; Germani, M.; Guanci, M.; et al. NMS-E973, a Novel Synthetic Inhibitor of Hsp90 with Activity against Multiple Models of Drug Resistance to Targeted Agents, Including Intracranial Metastases. Clin. Cancer Res. 2013, 19, 3520–3532. [Google Scholar] [CrossRef]
- Whitesell, L.; Shifrin, S.D.; Schwab, G.; Neckers, L.M. Benzoquinonoid ansamycins possess selective tumoricidal activity unrelated to src kinase inhibition. Cancer Res. 1992, 52, 1721–1728. [Google Scholar]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; E Myers, C.; Neckers, L.M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: Essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef]
- Neckers, L.; Workman, P. Hsp90 Molecular Chaperone Inhibitors: Are We There Yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef]
- Khandelwal, A.; Crowley, V.M.; Blagg, B.S.J. Natural Product Inspired N-Terminal Hsp90 Inhibitors: From Bench to Bedside? Med. Res. Rev. 2015, 36, 92–118. [Google Scholar] [CrossRef]
- Schnur, R.C.; Corman, M.L.; Gallaschun, R.J.; Cooper, B.A.; Dee, M.F.; Doty, J.L.; Muzzi, M.L.; Moyer, J.D.; DiOrio, C.I. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J. Med. Chem. 1995, 38, 3806–3812. [Google Scholar] [CrossRef]
- Rastelli, G.; Tian, Z.-Q.; Wang, Z.; Myles, D.; Liu, Y. Structure-based design of 7-carbamate analogs of geldanamycin. Bioorganic Med. Chem. Lett. 2005, 15, 5016–5021. [Google Scholar] [CrossRef]
- Kang, B.H.; Siegelin, M.D.; Plescia, J.; Raskett, C.M.; Garlick, D.S.; Dohi, T.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Preclinical Characterization of Mitochondria-Targeted Small Molecule Hsp90 Inhibitors, Gamitrinibs, in Advanced Prostate Cancer. Clin. Cancer Res. 2010, 16, 4779–4788. [Google Scholar] [CrossRef]
- Park, H.-K.; Lee, J.-E.; Lim, J.; Jo, D.-E.; Park, S.-A.; Suh, P.-G.; Kang, B.H. Combination treatment with doxorubicin and gamitrinib synergistically augments anticancer activity through enhanced activation of Bim. BMC Cancer 2014, 14, 431. [Google Scholar] [CrossRef]
- Kim, H.; Yang, J.; Kim, M.J.; Choi, S.; Chung, J.-R.; Kim, J.-M.; Yoo, Y.H.; Chung, J.; Koh, H. Tumor Necrosis Factor Receptor-associated Protein 1 (TRAP1) Mutation and TRAP1 Inhibitor Gamitrinib-triphenylphosphonium (G-TPP) Induce a Forkhead Box O (FOXO)-dependent Cell Protective Signal from Mitochondria. J. Biol. Chem. 2016, 291, 1841–1853. [Google Scholar] [CrossRef]
- Hayat, U.; Elliott, G.T.; Olszanski, A.J.; Altieri, D.C. Feasibility and safety of targeting mitochondria for cancer therapy—Preclinical characterization of gamitrinib, a first-in-class, mitochondriaL-targeted small molecule Hsp90 inhibitor. Cancer Biol. Ther. 2022, 23, 117–126. [Google Scholar] [CrossRef]
- Delmotte, P.; Delmotte-Plaquee, J. A New Antifungal Substance of Fungal Origin. Nature 1953, 171, 344. [Google Scholar] [CrossRef]
- Soga, S.; Shiotsu, Y.; Akinaga, S.; Sharma, S. Development of Radicicol Analogues. Curr. Cancer Drug Targets 2003, 3, 359–369. [Google Scholar] [CrossRef]
- Duerfeldt, A.S.; Brandt, G.E.L.; Blagg, B.S.J. Design, Synthesis, and Biological Evaluation of Conformationally Constrained cis-Amide Hsp90 Inhibitors. Org. Lett. 2009, 11, 2353–2356. [Google Scholar] [CrossRef]
- Soga, S.; Neckers, L.M.; Schulte, T.W.; Shiotsu, Y.; Akasaka, K.; Narumi, H.; Agatsuma, T.; Ikuina, Y.; Murakata, C.; Tamaoki, T.; et al. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res. 1999, 59, 2931–2938. [Google Scholar]
- Roe, S.M.; Prodromou, C.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Structural Basis for Inhibition of the Hsp90 Molecular Chaperone by the Antitumor Antibiotics Radicicol and Geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef]
- Proisy, N.; Sharp, S.Y.; Boxall, K.; Connelly, S.; Roe, M.; Prodromou, C.; Slawin, A.; Pearl, L.; Workman, P.; Moody, C. Inhibition of Hsp90 with Synthetic Macrolactones: Synthesis and Structural and Biological Evaluation of Ring and Conformational Analogs of Radicicol. Chem. Biol. 2006, 13, 1203–1215. [Google Scholar] [CrossRef]
- Moulin, E.; Zoete, V.; Barluenga, S.; Karplus, M.; Winssinger, N. Design, Synthesis, and Biological Evaluation of HSP90 Inhibitors Based on Conformational Analysis of Radicicol and Its Analogues. J. Am. Chem. Soc. 2005, 127, 6999–7004. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta 2012, 1823, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Perera, S.A.; Foley, K.P.; Sang, J.; Rodig, S.J.; Inoue, T.; Chen, L.; Li, D.; Carretero, J.; Li, Y.C.; et al. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin. Cancer Res. 2012, 18, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Du, Z.; Sun, L.; Foley, K.P.; Proia, D.A.; Blackman, R.K.; Zhou, D.; Inoue, T.; Tatsuta, N.; Sang, J.; et al. Ganetespib, a Unique Triazolone-Containing Hsp90 Inhibitor, Exhibits Potent Antitumor Activity and a Superior Safety Profile for Cancer Therapy. Mol. Cancer Ther. 2012, 11, 475–484. [Google Scholar] [CrossRef]
- Cheung, K.-M.J.; Matthews, T.P.; James, K.; Rowlands, M.G.; Boxall, K.J.; Sharp, S.Y.; Maloney, A.; Roe, S.M.; Prodromou, C.; Pearl, L.H.; et al. The identification, synthesis, protein crystal structure and in vitro biochemical evaluation of a new 3,4-diarylpyrazole class of Hsp90 inhibitors. Bioorganic Med. Chem. Lett. 2005, 15, 3338–3343. [Google Scholar] [CrossRef]
- Garon, E.B.; Finn, R.S.; Hamidi, H.; Dering, J.; Pitts, S.; Kamranpour, N.; Desai, A.J.; Hosmer, W.; Ide, S.; Avsar, E.; et al. The HSP90 Inhibitor NVP-AUY922 Potently Inhibits Non-Small Cell Lung Cancer Growth. Mol. Cancer Ther. 2013, 12, 890–900. [Google Scholar] [CrossRef]
- Ueno, T.; Tsukuda, K.; Toyooka, S.; Ando, M.; Takaoka, M.; Soh, J.; Asano, H.; Maki, Y.; Muraoka, T.; Tanaka, N.; et al. Strong anti-tumor effect of NVP-AUY922, a novel Hsp90 inhibitor, on non-small cell lung cancer. Lung Cancer 2011, 76, 26–31. [Google Scholar] [CrossRef]
- Perez, C.A.; Velez, M.; Raez, L.E.; Santos, E.S. Overcoming the resistance to crizotinib in patients with non-small cell lung cancer harboring EML4/ALK translocation. Lung Cancer. 2014, 84, 110–115. [Google Scholar] [CrossRef]
- Cavenagh, J.; Oakervee, H.; Baetiong-Caguioa, P.; Davies, F.; Gharibo, M.; Rabin, N.; Kurman, M.; Novak, B.; Shiraishi, N.; Nakashima, D.; et al. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br. J. Cancer 2017, 117, 1295–1302. [Google Scholar] [CrossRef]
- Smyth, T.; Van Looy, T.; Curry, J.E.; Rodriguez-Lopez, A.M.; Wozniak, A.; Zhu, M.; Donsky, R.; Morgan, J.G.; Mayeda, M.; Fletcher, J.A.; et al. The HSP90 Inhibitor, AT13387, Is Effective against Imatinib-Sensitive and -Resistant Gastrointestinal Stromal Tumor Models. Mol. Cancer Ther. 2012, 11, 1799–1808. [Google Scholar] [CrossRef]
- Clevenger, R.C.; Blagg, B.S.J. Design, Synthesis, and Evaluation of a Radicicol and Geldanamycin Chimera, Radamide. Org. Lett. 2004, 6, 4459–4462. [Google Scholar] [CrossRef]
- Shen, G.; Blagg, B.S.J. Radester, a Novel Inhibitor of the Hsp90 Protein Folding Machinery. Org. Lett. 2005, 7, 2157–2160. [Google Scholar] [CrossRef]
- Chiosis, G.; Lucas, B.; Shtil, A.; Huezo, H.; Rosen, N. Development of a Purine-Scaffold Novel Class of Hsp90 Binders that Inhibit the Proliferation of Cancer Cells and Induce the Degradation of Her2 Tyrosine Kinase. Bioorganic Med. Chem. 2002, 10, 3555–3564. [Google Scholar] [CrossRef]
- Chiosis, G.; Timaul, M.N.; Lucas, B.; Munster, P.N.; Zheng, F.F.; Sepp-Lorenzino, L.; Rosen, N. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem. Biol. 2001, 8, 289–299. [Google Scholar] [CrossRef]
- Llauger, L.; He, H.; Kim, J.; Aguirre, J.; Rosen, N.; Peters, U.; Davies, A.P.; Chiosis, G. Evaluation of 8-Arylsulfanyl, 8-Arylsulfoxyl, and 8-Arylsulfonyl Adenine Derivatives as Inhibitors of the Heat Shock Protein 90. J. Med. Chem. 2005, 48, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Okuno, S.H.; Keohan, M.L.; Maki, R.G.; D’Adamo, D.R.; Akhurst, T.J.; Antonescu, C.R.; Schwartz, G.K. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann. Oncol. 2012, 24, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P. Panobinostat: First Global Approval. Drugs 2015, 75, 695–704. [Google Scholar] [CrossRef]
- Seo, Y.H. Repositioning Irsogladine to Hsp90 Inhibitor. Bull. Korean Chem. Soc. 2015, 36, 1495–1499. [Google Scholar] [CrossRef]
- Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205353s000lbl.pdf (accessed on 30 July 2022).
- Mandawat, A.; Fiskus, W.; Buckley, K.M.; Robbins, K.; Rao, R.; Balusu, R.; Navenot, J.-M.; Wang, Z.-X.; Ustun, C.; Chong, D.G.; et al. Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood 2010, 116, 5306–5315. [Google Scholar] [CrossRef]
- Bots, M.; Verbrugge, I.; Martin, B.P.; Salmon, J.; Ghisi, M.; Baker, A.; Stanley, K.; Shortt, J.; Ossenkoppele, G.J.; Zuber, J.; et al. Differentiation therapy for the treatment of t(8;21) acute myeloid leukemia using histone deacetylase inhibitors. Blood 2014, 123, 1341–1352. [Google Scholar] [CrossRef]
- Imai, Y.; Ohta, E.; Takeda, S.; Sunamura, S.; Ishibashi, M.; Tamura, H.; Wang, Y.-H.; Deguchi, A.; Tanaka, J.; Maru, Y.; et al. Histone deacetylase inhibitor panobinostat induces calcineurin degradation in multiple myeloma. JCI Insight 2016, 1, e85061. [Google Scholar] [CrossRef]
- Ueda, F.; Aratani, S.; Mimura, K.; Kimura, K.; Nomura, A.; Enomoto, H. Effect of 2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate (MN-1695) on gastric ulcers and gastric secretion in experimental animals. Arzneim.-Forsch. 1984, 34, 314–320. [Google Scholar]
- Murakami, K.; Okimoto, T.; Kodama, M.; Tanahashi, J.; Mizukami, K.; Shuto, M.; Abe, H.; Arita, T.; Fujioka, T. Comparison of the efficacy of irsogladine maleate and famotidine for the healing of gastric ulcers after Helicobacter pylori eradication therapy: A randomized, controlled, prospective study. Scand J. Gastroenterol. 2011, 46, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Henry, E.C.; Gasiewicz, T.A. (−)-Epigallocatechin-3-gallate Is a Novel Hsp90 Inhibitor. Biochemistry 2008, 48, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Hall, J.A.; Blagg, B.S. Synthesis and structure-activity relationships of EGCG analogues, a recently identified Hsp90 inhibitor. J. Org. Chem. 2013, 78, 7859–7884. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Singh, R.P.; Dhanalakshmi, S.; Tyagi, A.K.; Tecklenburg, M.; A Sclafani, R.; Agarwal, R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene 2003, 22, 8271–8282. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Brandt, G.E.; Galam, L.; Matts, R.L.; Blagg, B.S. Identification and initial SAR of silybin: An Hsp90 inhibitor. Bioorganic Med. Chem. Lett. 2011, 21, 2659–2664. [Google Scholar] [CrossRef]
- Jordan, P.; Carmo-Fonseca, M. Molecular mechanisms involved in cisplatin cytotoxicity. Experientia 2000, 57, 1229–1235. [Google Scholar] [CrossRef]
- Itoh, H.; Ogura, M.; Komatsuda, A.; Wakui, H.; Miura, A.B.; Tashima, Y. A novel chaperone-activity-reducing mechanism of the 90-kDa molecular chaperone HSP90. Biochem. J. 1999, 343 Pt 3, 697–703. [Google Scholar] [CrossRef]
- Söti, C.; Rácz, A.; Csermely, P. A Nucleotide-dependent Molecular Switch Controls ATP Binding at the C-terminal Domain of Hsp90. J. Biol. Chem. 2002, 277, 7066–7075. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Gu, W.; Liu, S.; Silverman, R.B. Solid-Phase, Pd-Catalyzed Silicon-Aryl Carbon Bond Formation. Synthesis of Sansalvamide A Peptide. Org. Lett. 2002, 4, 4171–4174. [Google Scholar] [CrossRef]
- Kunicki, J.B.; Petersen, M.N.; Alexander, L.D.; Ardi, V.C.; McConnell, J.R.; McAlpine, S.R. Synthesis and evaluation of biotinylated sansalvamide A analogs and their modulation of Hsp90. Bioorg. Med. Chem. Lett. 2011, 21, 4716–4719. [Google Scholar] [CrossRef]
- Chang, D.-J.; An, H.; Kim, K.-S.; Kim, H.H.; Jung, J.; Lee, J.M.; Kim, N.-J.; Han, Y.T.; Yun, H.; Lee, S.; et al. Design, Synthesis, and Biological Evaluation of Novel Deguelin-Based Heat Shock Protein 90 (HSP90) Inhibitors Targeting Proliferation and Angiogenesis. J. Med. Chem. 2012, 55, 10863–10884. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Woo, J.K.; Yazici, Y.D.; Myers, J.N.; Kim, W.-Y.; Jin, Q.; Hong, S.S.; Park, H.-J.; Suh, Y.-G.; Kim, K.-W.; et al. Structural Basis for Depletion of Heat Shock Protein 90 Client Proteins by Deguelin. JNCI J. Natl. Cancer Inst. 2007, 99, 949–961. [Google Scholar] [CrossRef]
- Caboni, P.; Sherer, T.B.; Zhang, N.; Taylor, G.; Na, H.M.; Greenamyre, J.T.; Casida, J.E. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem Res. Toxicol. 2004, 17, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D.; Yan, P.; Seidler, P.M.; Patel, H.J.; Sun, W.; Yang, C.; Que, N.; Taldone, T.; Finotti, P.; Stephani, R.A.; et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 2013, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Álvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Zhou, G.-B. The Main Anticancer Bullets of the Chinese Medicinal Herb, Thunder God Vine. Molecules 2011, 16, 5283–5297. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Y.; Li, R.; Liu, Y.; Wen, L.; Zhang, C. Triptolide alters histone H3K9 and H3K27 methylation state and induces G0/G1 arrest and caspase-dependent apoptosis in multiple myeloma in vitro. Toxicology 2010, 267, 70–79. [Google Scholar] [CrossRef]
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Dou, Q.P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine”, is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Huang, Y.-L. Antiangiogenic effect of celastrol on the growth of human glioma: An in vitro and in vivo study. Chin. Med. J. 2009, 122, 1666–1673. [Google Scholar]
- Sreeramulu, S.; Gande, S.L.; Göbel, M.; Schwalbe, H. Molecular Mechanism of Inhibition of the Human Protein Complex Hsp90-Cdc37, a Kinome Chaperone-Cochaperone, by Triterpene Celastrol. Angew. Chem. Int. Ed. 2009, 48, 5853–5855. [Google Scholar] [CrossRef]
- Zhang, T.; Hamza, A.; Cao, X.; Wang, B.; Yu, S.; Zhan, C.G.; Sun, D. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 162–170. [Google Scholar] [CrossRef]
- Patwardhan, C.A.; Fauq, A.; Peterson, L.B.; Miller, C.; Blagg, B.S.J.; Chadli, A. Gedunin Inactivates the Co-chaperone p23 Protein Causing Cancer Cell Death by Apoptosis. J. Biol. Chem. 2013, 288, 7313–7325. [Google Scholar] [CrossRef]
- Kaileh, M.; Vanden Berghe, W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; De Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef]
- Falsey, R.R.; Marron, M.T.; Gunaherath, G.M.K.B.; Shirahatti, N.; Mahadevan, D.; Gunatilaka, A.A.L.; Whitesell, L. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat. Chem. Biol. 2005, 2, 33–38. [Google Scholar] [CrossRef]
- Shohat, B.; Gitter, S.; Abraham, A.; Lavie, D. Antitumor activity of withaferin A (NSC-101088). Cancer Chemother. Rep. 1967, 51, 271–276. [Google Scholar]
- Srinivasan, S.; Ranga, R.S.; Burikhanov, R.; Han, S.-S.; Chendil, D. Par-4-Dependent Apoptosis by the Dietary Compound Withaferin A in Prostate Cancer Cells. Cancer Res. 2007, 67, 246–253. [Google Scholar] [CrossRef]
- Hadden, M.K.; Galam, L.; Gestwicki, J.E.; Matts, R.L.; Blagg, B.S.J. Derrubone, an Inhibitor of the Hsp90 Protein Folding Machinery. J. Nat. Prod. 2007, 70, 2014–2018. [Google Scholar] [CrossRef]
- Hastings, J.M.; Hadden, A.M.K.; Blagg, B.S.J. Synthesis and Evaluation of Derrubone and Select Analogues. J. Org. Chem. 2007, 73, 369–373. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, C.; Chai, H.B.; Ding, Y.; Li, X.C.; Ferreira, D.; Kinghorn, A.D. Absolute configuration of (−)-gambogic acid, an antitumor agent. J. Nat. Prod. 2011, 74, 460–463. [Google Scholar] [CrossRef]
- Chi, Y.; Zhan, X.-K.; Yu, H.; Xie, G.-R.; Wang, Z.-Z.; Xiao, W.; Wang, Y.-G.; Xiong, F.-X.; Hu, J.-F.; Yang, L.; et al. An open-labeled, randomized, multicenter phase IIa study of gambogic acid injection for advanced malignant tumors. Chin. Med. J. 2013, 126, 1642–1646. [Google Scholar]
- Kunze, B.; Sasse, F.; Wieczorek, H.; Huss, M. Cruentaren A, a highly cytotoxic benzolactone from Myxobacteria is a novel selective inhibitor of mitochondrial F1-ATPases. FEBS Lett. 2007, 581, 3523–3527. [Google Scholar] [CrossRef]
- Kunze, B.; Steinmetz, H.; Höfle, G.; Huss, M.; Wieczorek, H.; Reichenbach, H. Cruentaren, a New Antifungal Salicylate-Type Macrolide from Byssovorax cruenta (Myxobacteria) with Inhibitory Effect on Mitochondrial ATPase Activity. J. Antibiot. 2006, 59, 664–668. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, F.; Yang, C.; Wang, L.; Sung, J.; Garg, P.; Zhang, M.; Merlin, D. Oral Targeted Delivery by Nanoparticles Enhances Efficacy of an Hsp90 Inhibitor by Reducing Systemic Exposure in Murine Models of Colitis and Colitis-Associated Cancer. J. Crohn’s Colitis 2019, 14, 130–141. [Google Scholar] [CrossRef]
- Smalley, M.; Natarajan, S.K.; Mondal, J.; Best, D.; Goldman, D.; Shanthappa, B.; Pellowe, M.; Dash, C.; Saha, T.; Khiste, S.; et al. Nanoengineered Disruption of Heat Shock Protein 90 Targets Drug-Induced Resistance and Relieves Natural Killer Cell Suppression in Breast Cancer. Cancer Res. 2020, 80, 5355–5366. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Guo, W.; Long, Q.; Ma, A.; Liu, Q.; Zhang, H.; Huang, Y.; Chandrasekaran, S.; Pan, C.; Lam, K.S.; et al. HSP90 Inhibitor Encapsulated Photo-Theranostic Nanoparticles for Synergistic Combination Cancer Therapy. Theranostics 2016, 6, 1324–1335. [Google Scholar] [CrossRef]
- Rochani, A.K.; Balasubramanian, S.; Girija, A.R.; Maekawa, T.; Kaushal, G.; Kumar, D.S. Heat Shock Protein 90 (Hsp90)-Inhibitor-Luminespib-Loaded-Protein-Based Nanoformulation for Cancer Therapy. Polymers 2020, 12, 1798. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, B.; Wales, C.T.; Taylor, F.R.; Jacobs, A.T. Heat shock factor 1 confers resistance to Hsp90 inhibitors through p62/SQSTM1 expression and promotion of autophagic flux. Biochem. Pharmacol. 2014, 87, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Loo, A.; Jaeger, S.; Bagdasarian, L.; Yu, J.; Chung, F.; Korn, J.; Ruddy, D.; Guo, R.; et al. Targeting HSF1 sensitizes cancer cells to HSP90 inhibition. Oncotarget 2013, 4, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.; Sharp, S.Y.; Pacey, S.; Jones, C.; Walton, M.; Vassal, G.; Eccles, S.; Pearson, A.; Workman, P. Acquired Resistance to 17-Allylamino-17-Demethoxygeldanamycin (17-AAG, Tanespimycin) in Glioblastoma Cells. Cancer Res. 2009, 69, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Blower, P.E.; Pham, A.-N.; Fang, J.; Dai, Z.; Wise, C.; Green, B.; Teitel, C.H.; Ning, B.; Ling, W.; et al. Cystine-Glutamate Transporter SLC7A11 Mediates Resistance to Geldanamycin but Not to 17-(Allylamino)-17-demethoxygeldanamycin. Mol. Pharmacol. 2007, 72, 1637–1646. [Google Scholar] [CrossRef]
- Yuno, A.; Lee, M.-J.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J.B. Clinical Evaluation and Biomarker Profiling of Hsp90 Inhibitors. Chaperones 2017, 1709, 423–441. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toft, D.; Reid, J.; Ames, M.; Stensgard, B.; Safgren, S.; Adjei, A.A.; Sloan, J.; Atherton, P.; Vasile, V.; et al. Phase I Trial of 17-Allylamino-17-Demethoxygeldanamycin in Patients with Advanced Cancer. J. Clin. Oncol. 2005, 23, 1078–1087. [Google Scholar] [CrossRef]
- Solit, D.B.; Ivy, S.P.; Kopil, C.; Sikorski, R.; Morris, M.J.; Slovin, S.F.; Kelly, W.K.; DeLaCruz, A.; Curley, T.; Heller, G.; et al. Phase I Trial of 17-Allylamino-17-Demethoxygeldanamycin in Patients with Advanced Cancer. Clin. Cancer Res. 2007, 13, 1775–1782. [Google Scholar] [CrossRef]
- Peron, M.; Bonvini, P.; Rosolen, A. Effect of inhibition of the Ubiquitin-Proteasome System and Hsp90 on growth and survival of Rhabdomyosarcoma cells in vitro. BMC Cancer 2012, 12, 233. [Google Scholar] [CrossRef]
- O’Connell, B.C.; O’Callaghan, K.; Tillotson, B.; Douglas, M.; Hafeez, N.; West, K.A.; Stern, H.; Ali, J.A.; Changelian, P.; Fritz, C.C.; et al. HSP90 Inhibition Enhances Antimitotic Drug-Induced Mitotic Arrest and Cell Death in Preclinical Models of Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e115228. [Google Scholar] [CrossRef]
- Wong, D.S.; Jay, D.G. Emerging Roles of Extracellular Hsp90 in Cancer, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 129. [Google Scholar] [CrossRef]
- Li, W.; Tsen, F.; Sahu, D.; Bhatia, A.; Chen, M.; Multhoff, G.; Woodley, D.T. Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: Intentionally or unintentionally. Int. Rev. Cell Mol. Biol. 2013, 303, 203–235. [Google Scholar]


| Phenotype | Clients | References |
|---|---|---|
| Uncontrolled proliferation | EGFR, HER2, RAF1, CDK4, Akt, BCR-ABL, v-Src, c-Src, FAK, CKII, CHK1, eIF-2α kinase | [6,16,17,54,55,56,57,58,59,60,61,62] |
| Anti-apoptosis | p53, Akt, Survivin, IKK, NF-κB, PLK, WEE1, Myc, CDK4, CDK6 | [6,42,55,63,64,65,66,67,68] |
| Angiogenesis | HIF1α, Akt, EGFR, HRE2, FLT3, VEGFR2 | [19,69,70,71,72,73] |
| Immortalization | Telomerase | [18] |
| Invasion/Metastasis | MMP2, MMP9, c-MET | [19,34,74,75] |
| Others | Glucocorticoid receptor, Mineralocorticoid receptor, Progesterone receptor, Estrogen receptor, Androgen receptor, Oestrogen receptor, Nitric oxide synthase, Centrin/centrosome, Calmodulin, MDM2, UHRF1, BRCA2, OCT4, Nanog, STAT3, Calcineurin, CFTR, NLR proteins, RAD51/RAD52, Tau, HCK, JAK1 and/or JAK2 | [76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] |
| Type | Order | Inhibitor | Alias/Description | Structure | HSP90 Binding Site | Organelle selectivity | Manufacturer | Application | Drug Used for Combination | Recruting or Active Clinical Stage | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA and its derivatives | 1 | Geldanamycin | GA, NSC 122750 |  | N-terminal ATP-binding pocket | NCI | melanoma, leukemia, colorectal cancer, prostate cancer, lung cancer, breast cancer, kidney cancer, bladder cancer, gastric cancer, head and neck cancer, ovarian cancer, neuroblastoma, osteosarcoma | docetaxel, irinotecan hydrochloride, | Phase I/II | [126,127] | |
| 2 | 17- AAG | Tanespimycin |  | N-terminal ATP-binding pocket | Cytosol | Pfizer | thyroid cancer, lymphoma, leukemia, prostate cancer, neuroblastoma, osteosarcoma, sarcoma, lung cancer, myeloma, kidney cancer, ovarian epithelial cancer, pancreatic cancer, breast cancer | irinotecan hydrochloride, sorafenib tosylate, cytarabine, docetaxel, gemcitabine hydrochloride, everolimus, Bortezomib | Phase I/II/ III | [128,129] | |
| 3 | 17- DMAG | Alvespimycin |  | N-terminal ATP-binding pocket | NCI | lymphoma, breast cancer, lung cancer, gastric cancer, prostate cancer, myeloma | Trastuzumab | Phase I | [130,131,132] | ||
| 4 | IPI-504 | Retaspimycin |  | N-terminal ATP-binding pocket | Cytosol | Infinity | lung cancer, prostate cancer, myeloma, sarcoma, leukemia, lymphoma, pancreatic cancer, ovarian epithelial cancer, breast cancer, kidney cancer, bladder cancer, gastric cancer | docetaxel, everolimus, cytarabine, gemcitabine hydrochloride, irinotecan hydrochloride, sorafenib tosylate, Bortezomib, imatinib mesylate | Phase I/II/ III | [133] | |
| 5 | NVP-BEP800 | VER82576 |  | N-terminal ATP-binding pocket | [134,135] | ||||||
| 6 | TAS-116 | Pimitespib |  | N-terminal ATP-binding pocket | Cytosol | Taiho | Gastrointestinal cancer, pancreatic cancer, lung cancer, colorectal cancer | Phase I | [136,137] | ||
| 7 | Gamitrinib TPP |  | N-terminal ATP-binding pocket | Mitochondrion | lymphoma | Phase I | [138,139] | ||||
| 8 | SNX-2112 | PF-04928473 |  | N-terminal ATP-binding pocket | Serenex | [140] | |||||
| 9 | Macbecin |  | N-terminal ATP-binding pocket | [141] | |||||||
| 10 | XL888 |  | N-terminal ATP-binding pocket | Exelixis | colorectal cancer, myeloma, pancreatic cancer | Vemurafenib, Cobimetinib | Phase I | [142,143,144] | |||
| Radicicol or resorcinol-containing derivatives | 11 | Radicicol | RA, RDC |  | N-terminal ATP-binding pocket | [127,145,146] | |||||
| 12 | STA-9090 | Ganetespib |  | N-terminal ATP-binding pocket | Synta Pharmaceuticals | Hepatocellular Carcinoma, Esophagogastric Cancer, melanoma, breast cacncer, lung cancer, colorectal cancer, prostate cancer, leukemia, myeloma, ovarian cancer | Docetaxel, crizotinib, Sirolimus, capecitabine, Bortezomib, Dexamethasone, Fulvestrant, Carboplatin | Phase I/II | [147,148] | ||
| 13 | CCT018159 |  | N-terminal ATP-binding pocket | [149,150] | |||||||
| 14 | NVP-AUY992 | Isoxazole/luminespib |  | N-terminal ATP-binding pocket | Novartis | [151] | |||||
| 15 | KW-2478 |  | unknown | Kyowa Hakko Kirin | Bortezomib | Phase I/II | [152] | ||||
| 16 | AT13387 |  | N-terminal ATP-binding pocket | Astex Pharmaceuticals | Gastrointestinal Stromal Tumors, pancreatic cancer, lung cancer, breast cancer | Crizotinib, Imatinib, abiraterone acetate, Prednisone, Onalespib | Phase I/II | [153,154] | |||
| GA and RA chimeric molecules | 17 | Radanamycin |  | N-terminal ATP-binding pocket | [155] | ||||||
| 18 | Radamide |  | N-terminal ATP-binding pocket | [155] | |||||||
| 19 | Radester |  | N-terminal ATP-binding pocket | [155] | |||||||
| Purine-based molecules | 20 | PU3 |  | N-terminal ATP-binding pocket | Phase I | [156] | |||||
| 21 | BIIB021 | CNF2024 |  | N-terminal ATP-binding pocket | Biogen Idec | breast cancer, lymphoma | exemestane (Aromasin), trastuzumab | Phase I/II | [157,158] | ||
| 22 | BIIB028 |  | N-terminal ATP-binding pocket | Conforma Therapeutics | breast cancer, melanoma, gastrointestinal cancer, lymphoma, myeloma | Onalespib, Bortezomib, Cetuximab | Phase I/II/ III | [159] | |||
| 23 | DN401 |  | N-terminal ATP-binding pocket | [9,160] | |||||||
| 24 | MPC-3100 |  | N-terminal ATP-binding pocket | Myriad Pharmaceuticals | Phase I | [161] | |||||
| Other inhibitors | 25 | Panobinostat | Farydak |  | Novartis | Phase I/II/ III | |||||
| 26 | Novobiocin | Albamycin/ cathomycin |  | C-terminus | Pharmacia & Upjohn | [162] | |||||
| 27 | EGCG | Epigallocatechin gallate |  | C-terminus | colon cancer, prostate cancer, bladder cancer, head and neck cancer, breast cancer, lung cancer, pancreatic cancer | Sunphenon, Erlotinib, Polyphenon E | Phase I/II/ III/IV | [163,164] | |||
| 28 | Silybin |  | C-terminus | prostate cancer | Phase I/II/ III/IV | [165] | |||||
| 29 | Cisplatin |  | C-terminus | Phase I/II/ III/IV | [166,167,168] | ||||||
| 30 | LA-12 | Cisplatin derivative |  | C-terminus | Phase I/II/ III/IV | [168,169,170] | |||||
| 31 | Paclitaxel | Taxol |  | unkonwn | Phase I/II/ III/IV | [171] | |||||
| 32 | Sansalvamide A | San A |  | N-MD | [172,173] | ||||||
| 33 | L80 | Deguelin derivative |  | C-terminal ATP-binding pocket | Phase III | [174] | |||||
| 34 | Shepherdin | / |  | N-terminal ATP-binding pocket | Mitochondrion | [26,175] | |||||
| 35 | SNX-5422 | PF-04929113 |  | N-terminal ATP-binding pocket | Pfizer | leukemia, lymphoma. | Phase I | [176,177] | |||
| 36 | HSP990 | NVP- HSP990 |  | N-terminal ATP-binding pocket | Novartis | Phase I | [178] | ||||
| 37 | Pseudolaric Acid A | PAA |  | N-terminal ATP-binding pocket | [179] | ||||||
| 38 | CH5138303 |  | N-terminal ATP-binding pocket | [180] | |||||||
| 39 | NMS-E973 | Isoxazole derivative |  | N-terminal ATP-binding pocket | Nerviano Medical Sciences S.r.l. laboratories | [181] |
| Drug | Number of Clinical Trials |
|---|---|
| Bortezomib | 5 |
| Docetaxel | 4 |
| Irinotecan | 3 |
| Sorafenib | 2 |
| Cytarabine | 2 |
| Gemcitabine | 2 |
| Everolimus | 2 |
| Trastuzumab | 2 |
| Imatinib | 2 |
| Crizotinib | 2 |
| Onalespib | 2 |
| Vemurafenib | 1 |
| Cobimetinib | 1 |
| Sirolimus | 1 |
| Capecitabine | 1 |
| Dexamethasone | 1 |
| Fulvestrant | 1 |
| Carboplatin | 1 |
| Abiraterone | 1 |
| Prednisone | 1 |
| Exemestane (Aromasin) | 1 |
| Cetuximab | 1 |
| Sunphenon | 1 |
| Erlotinib | 1 |
| Polyphenon E | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, H.; Liu, Y.; Zhao, K.; Wei, S.; Sugarman, E.T.; Liu, L.; Zhang, G. Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance. Cells 2022, 11, 2778. https://doi.org/10.3390/cells11182778
Zhang J, Li H, Liu Y, Zhao K, Wei S, Sugarman ET, Liu L, Zhang G. Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance. Cells. 2022; 11(18):2778. https://doi.org/10.3390/cells11182778
Chicago/Turabian StyleZhang, Jian, Houde Li, Yu Liu, Kejia Zhao, Shiyou Wei, Eric T. Sugarman, Lunxu Liu, and Gao Zhang. 2022. "Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance" Cells 11, no. 18: 2778. https://doi.org/10.3390/cells11182778
APA StyleZhang, J., Li, H., Liu, Y., Zhao, K., Wei, S., Sugarman, E. T., Liu, L., & Zhang, G. (2022). Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance. Cells, 11(18), 2778. https://doi.org/10.3390/cells11182778