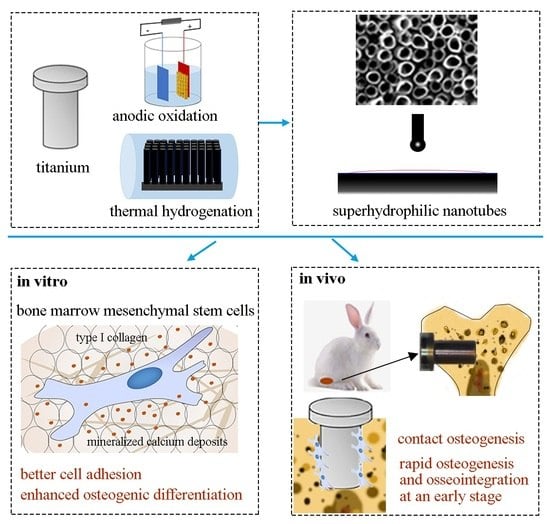
In Vitro and In Vivo Studies of Hydrogenated Titanium Dioxide Nanotubes with Superhydrophilic Surfaces during Early Osseointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. In Vitro Studies
2.2.1. Cell Adhesion and Proliferation Assays
2.2.2. Cell Morphology
2.2.3. Alkaline Phosphatase (ALP) Activity Assay
2.2.4. In Vitro Mineralization
2.2.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.3. In Vivo Studies
2.3.1. Implants
2.3.2. Animals
2.3.3. Surgical Procedures
2.3.4. Micro-Computed Tomography (Micro-CT)
2.3.5. Histology
2.3.6. Histometric Analysis
2.4. Statistical Analysis
3. Results
3.1. In Vitro Studies
3.1.1. Surface Characteristics
3.1.2. BMSC Adhesion and Proliferation
3.1.3. Osteogenic Differentiation of BMSCs
3.1.4. Osteogenic-Related Gene Expression
3.2. In Vivo Studies
3.2.1. Micro-CT Analysis
3.2.2. MAR Measurement
3.2.3. Histological Examination
3.2.4. Histometric Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.B.; Slosar, P.; Schwartz, Z.; Cohen, D.J.; Goodman, S.B.; Anderson, P.A.; Boyan, B.D. A review of biomimetic topographies and their role in promoting bone formation and osseointegration: Implications for clinical use. Biomimetics 2022, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, J.; He, Y.; Su, J.; Xu, L.; Zeng, X. Fabrication of an ordered micro-/nanotextured titanium surface to improve osseointegration. Colloids Surf B Biointerfaces 2022, 214, 112446. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhou, M.; Chen, W.; Zhu, Y.; Wang, X.; Zhang, Y.; Zhang, Q. Bioinspired polydopamine/graphene oxide/collagen nanofilms as a controlled release carrier of bioactive substances. Chem. Eng. J. 2021, 405, 126930. [Google Scholar] [CrossRef]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef]
- Hou, C.; An, J.; Zhao, D.; Ma, X.; Zhang, W.; Zhao, W.; Wu, M.; Zhang, Z.; Yuan, F. Surface modification techniques to produce micro/nano-scale topographies on Ti-based implant surfaces for improved osseointegration. Front. Bioeng. Biotechnol. 2022, 10, 835008. [Google Scholar] [CrossRef]
- Vordemvenne, T.; Wahnert, D.; Koettnitz, J.; Merten, M.; Fokin, N.; Becker, A.; Büker, B.; Vogel, A.; Kronenberg, D.; Stange, R.; et al. Bone regeneration: A novel osteoinductive function of spongostan by the interplay between its nano- and microtopography. Cells 2020, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalinska, A.; Chodara, A.; Szalaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun membrane surface modification by sonocoating with HA and ZnO:Ag nanoparticles-Characterization and evaluation of osteoblasts and bacterial cell behavior in vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Nanda, A.; Walsh, L.J.; Xu, C. Microbial decontamination and antibacterial activity of nanostructured titanium dental implants: A narrative review. Nanomaterials 2021, 11, 2336. [Google Scholar] [CrossRef]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100-B, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jayasree, A.; Ivanovski, S.; Gulati, K. ON or OFF: Triggered therapies from anodized nano-engineered titanium implants. J. Control. Release 2021, 333, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 2015, 39, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, S.; Chang, J.; Huan, Z.; Li, H. TiO2 nanotubes enhance vascularization and osteogenic differentiation through stimulating interactions between bone marrow stromal cells and endothelial cells. J. Biomed. Nanotechnol. 2018, 14, 765–777. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, X.; Luo, Z.; Hu, Y.; Li, M.; Ma, P.; Ran, Q.; Dai, L.; He, Y.; Cai, K. Osteogenesis potential of different titania nanotubes in oxidative stress microenvironment. Biomaterials 2018, 167, 44–57. [Google Scholar] [CrossRef]
- Baker, E.A.; Vara, A.D.; Salisbury, M.R.; Fleischer, M.M.; Baker, K.C.; Fortin, P.T.; Roberts, R.V.; Friedrich, C.R. Titania nanotube morphologies for osseointegration via models of in vitro osseointegrative potential and in vivo intramedullary fixation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1483–1493. [Google Scholar] [CrossRef]
- Gomez Sanchez, A.; Katunar, M.R.; Pastore, J.I.; Tano de la Hoz, M.F.; Cere, S. Evaluation of annealed titanium oxide nanotubes on titanium: From surface characterization to in vivo assays. J. Biomed. Mater. Res. A 2021, 109, 1088–1100. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Chen, P.; Zhang, X.; Huang, X.; Du, Z.; Crawford, R.; Yao, X.; Tang, B.; Hang, R.; et al. Targeting early healing phase with titania nanotube arrays on tunable diameters to accelerate bone regeneration and osseointegration. Small 2021, 17, e2006287. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Alves, A.; Zarranz, L.; Jorge, M.Z.; Granjeiro, J.; Calasans-Maia, M. Hydrophilic surface of Ti6Al4V-ELI alloy improves the early bone apposition of sheep tibia. Clin. Oral Implant. Res. 2017, 28, 893–901. [Google Scholar] [CrossRef]
- Cabrera-Domínguez, J.J.; Castellanos-Cosano, L.; Torres-Lagares, D.; Pérez-Fierro, M.; Machuca-Portillo, G. Clinical performance of titanium-zirconium implants with a hydrophilic surface in patients with controlled type 2 diabetes mellitus: 2-year results from a prospective case-control clinical study. Clin. Oral Investig. 2019, 24, 2477–2486. [Google Scholar] [CrossRef]
- Siqueira, R.; Ferreira, J.A.; Rizzante, F.A.P.; Moura, G.F.; Mendonça, D.B.S.; de Magalhães, D.; Cimões, R.; Mendonça, G. Hydrophilic titanium surface modulates early stages of osseointegration in osteoporosis. J. Periodontal Res. 2021, 56, 351–362. [Google Scholar] [CrossRef]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef]
- Gu, Y.-X.; Du, J.; Si, M.-S.; Mo, J.-J.; Qiao, S.-C.; Lai, H.-C. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J. Biomed. Mater. Res. A 2013, 101, 748–754. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef]
- Tsujita, H.; Nishizaki, H.; Miyake, A.; Takao, S.; Komasa, S. Effect of plasma treatment on titanium surface on the tissue surrounding implant material. Int. J. Mol. Sci. 2021, 22, 6931. [Google Scholar] [CrossRef]
- Shao, H.; Ma, M.; Wang, Q.; Yan, T.; Zhao, B.; Guo, S.; Tong, S. Advances in the superhydrophilicity-modified titanium surfaces with antibacterial and pro-osteogenesis properties: A review. Front. Bioeng. Biotechnol. 2022, 10, 1000401. [Google Scholar] [CrossRef]
- Hyzy, S.L.; Olivares-Navarrete, R.; Ortman, S.; Boyan, B.D.; Schwartz, Z. Bone morphogenetic protein 2 alters osteogenesis and anti-inflammatory profiles of mesenchymal stem cells induced by microtextured titanium in vitro. Tissue Eng. Part A 2017, 23, 1132–1141. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, J.; Zhang, L.; Xu, K. A multi-scaled hybrid orthopedic implant: Bone ECM-shaped Sr-HA nanofibers on the microporous walls of a macroporous titanium scaffold. Nanotechnology 2011, 22, 275603. [Google Scholar] [CrossRef] [Green Version]
- Leon-Ramos, J.R.; Diosdado-Cano, J.M.; Lopez-Santos, C.; Barranco, A.; Torres-Lagares, D.; Serrera-Figallo, M. Influence of titanium oxide pillar array nanometric structures and ultraviolet irradiation on the properties of the surface of dental implants—A pilot study. Nanomaterials 2019, 9, 1458. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Hartjen, P.; Heinrich, O.; Heuberger, R.; Heiland, M.; Precht, C.; Cacaci, C. Photofunctionalization and non-thermal plasma activation of titanium surfaces. Clin. Oral Investig. 2018, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.B.; Bosh, K.B.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Benchtop plasma treatment of titanium surfaces enhances cell response. Dent. Mater. 2021, 37, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to superhydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf B Biointerfaces 2021, 198, 111489. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lu, R.; Wang, X.; Chou, J.; Wang, N.; Huai, X.; Wang, C.; Zhao, Y.; Chen, S. Immune response of macrophages on super-hydrophilic TiO2 nanotube arrays. J. Biomater. Appl. 2020, 34, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wang, C.; Wang, X.; Wang, Y.; Wang, N.; Chou, J.; Li, T.; Zhang, Z.; Ling, Y.; Chen, S. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int. J. Nanomed. 2018, 13, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. EXCLI J. 2021, 20, 454–489. [Google Scholar] [PubMed]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Xu, H.; Jiang, Y.; Wang, D.; Liu, R. Influence of simvastatin-strontium-hydroxyapatite coated implant formed by micro-arc oxidation and immersion method on osteointegration in osteoporotic rabbits. Int. J. Nanomed. 2020, 15, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Boyan, B.D.; Lotz, E.M.; Schwartz, Z. Roughness and hydrophilicity as osteogenic biomimetic surface properties. Tissue Eng. Part A 2017, 23, 1479–1489. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1125–1142. [Google Scholar] [CrossRef]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.; Liu, Y.; Xia, R.; Chang, Y.; Hu, Y.; Liu, P.; Zhai, Z.; Zhang, J.; Li, H. F-actin regulates osteoblastic differentiation of mesenchymal stem cells on TiO2 nanotubes through MKL1 and YAP/TAZ. Nanoscale Res. Lett. 2020, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Wang, C.; Xia, R.; Li, H.; Yu, H.; Jing, J.; Cheng, W. TiO2 nanotubes regulate histone acetylation through F-actin to induce the osteogenic differentiation of BMSCs. Artif. Cells Nanomed. Biotechnol. 2021, 49, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Chang, Y.; Hu, Y.; Qiao, H.; Zhao, C.; Rong, K.; Zhang, P.; Zhang, J.; Zhai, Z.; Li, H. TiO2 nanotubes promote osteogenic differentiation through regulation of Yap and Piezo1. Front. Bioeng. Biotechnol. 2022, 10, 872088. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Vargas, A.L.M.; Sesterheim, P.; Teixeira, E.R.; Hubler, R. Extension of hydrophilicity stability by reactive plasma treatment and wet storage on TiO2 nanotube surfaces for biomedical implant applications. J. R. Soc. Interface 2020, 17, 20200650. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Zong, Z.; Ding, N.; Zhang, Z. Enhanced osteogenic activity of titania-modified zirconia implant by ultraviolet irradiation. Front. Bioeng. Biotechnol. 2022, 10, 945869. [Google Scholar] [CrossRef]
- Lotz, E.M.; Olivares-Navarrete, R.; Berner, S.; Boyan, B.D.; Schwartz, Z. Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J. Biomed. Mater. Res. A 2016, 104, 3137–3148. [Google Scholar] [CrossRef]
- Parisi, L.; Ghezzi, B.; Bianchi, M.G.; Toffoli, A.; Rossi, F.; Bussolati, O.; Macaluso, G.M. Titanium dental implants hydrophilicity promotes preferential serum fibronectin over albumin competitive adsorption modulating early cell response. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111307. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Toyoshima, T.; Götz, H.; Von Koppenfels, R.L.; Al-Nawas, B.; Duschner, H. Long-term response of osteogenic cells on micron and submicron-scale-structured hydrophilic titanium surfaces: Sequence of cell proliferation and cell differentiation. Clin. Oral Implants Res. 2010, 21, 642–649. [Google Scholar] [CrossRef]
- Ryoo, H.M.; Lee, M.H.; Kim, Y.J. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene 2006, 366, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gong, Y.; Xu, L.; Zhou, M.; Li, J.; Song, J. Bidirectional regulation of osteogenic differentiation by the FOXO subfamily of Forkhead transcription factors in mammalian MSCs. Cell Prolif. 2019, 52, e12540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ching, H.S.; Luddin, N.; Rahman, I.A.; Ponnuraj, K.T. Expression of odontogenic and osteogenic markers in DPSCs and SHED: A review. Curr. Stem Cell Res. Ther. 2017, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Brogini, S.; Sartori, M.; Giavaresi, G.; Cremascoli, P.; Alemani, F.; Bellini, D.; Martini, L.; Maglio, M.; Pagani, S.; Fini, M. Osseointegration of additive manufacturing Ti-6Al-4V and Co-Cr-Mo alloys, with and without surface functionalization with hydroxyapatite and type I collagen. J. Mech. Behav. Biomed. Mater. 2021, 115, 104262. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Zhao, B.; Jiang, S. Accelerated and enhanced osteointegration of MAO-treated implants: Histological and histomorphometric evaluation in a rabbit model. Int. J. Oral Sci. 2018, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Thiem, D.G.; Adam, M.; Ganz, C.; Gerber, T.; Kämmerer, P. The implant surface and its role in affecting the dynamic processes of bone remodeling by means of distance osteogenesis: A comparative in vivo study. Int. J. Oral Maxillofac. Implants 2019, 34, 133–140. [Google Scholar] [CrossRef]
- Takada, S.; Hirata, E.; Sakairi, M.; Miyako, E.; Takano, Y.; Ushijima, N.; Yudasaka, M.; Iijima, S.; Yokoyama, A. Carbon nanohorn coating by electrodeposition accelerate bone formation on titanium implant. Artif. Cells Nanomed. Biotechnol. 2021, 49, 20–29. [Google Scholar] [CrossRef]
- Khosravi, N.; DaCosta, R.S.; Davies, J.E. New insights into spatio-temporal dynamics of mesenchymal progenitor cell ingress during peri-implant wound healing: Provided by intravital imaging. Biomaterials 2021, 273, 120837. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tashiro, Y.; Komasa, S.; Miyake, A.; Komasa, Y.; Okazaki, J. Effects of surface modification on adsorption behavior of cell and protein on titanium surface by using quartz crystal microbalance system. Materials 2020, 14, 97. [Google Scholar] [CrossRef]
- Hyzy, S.L.; Cheng, A.; Cohen, D.J.; Yatzkaier, G.; Whitehead, A.J.; Clohessy, R.M.; Gittens, R.A.; Boyan, B.D.; Schwartz, Z. Novel hydrophilic nanostructured microtexture on direct metal laser sintered Ti-6Al-4V surfaces enhances osteoblast response in vitro and osseointegration in a rabbit model. J. Biomed. Mater. Res. A 2016, 104, 2086–2098. [Google Scholar] [CrossRef]






| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| ALP | GAACAGAACTGATGTGGAATACGAA | CAGTGCGGTTCCAGACATAGTG |
| COL-1 | GATGTTGAACTTGTTGTTGCTGAGGG | GGCAGGCGAGATGGCTTATT |
| OCN | TTCTGCTCACTCTGCTGACC | ACCACTCCAGCACAACTCCT |
| BMP-2 | TCCCCAGTGACGAGTTTCTC | GTCGAAGCTCTCCCACTGAC |
| RUNX2 | GCCGGGAATGATGAGAACTA | GGACCGTCCACTGTCACTTT |
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Gao, S.; Lu, R.; Wang, X.; Chen, S. In Vitro and In Vivo Studies of Hydrogenated Titanium Dioxide Nanotubes with Superhydrophilic Surfaces during Early Osseointegration. Cells 2022, 11, 3417. https://doi.org/10.3390/cells11213417
Wang C, Gao S, Lu R, Wang X, Chen S. In Vitro and In Vivo Studies of Hydrogenated Titanium Dioxide Nanotubes with Superhydrophilic Surfaces during Early Osseointegration. Cells. 2022; 11(21):3417. https://doi.org/10.3390/cells11213417
Chicago/Turabian StyleWang, Caiyun, Shang Gao, Ran Lu, Xin Wang, and Su Chen. 2022. "In Vitro and In Vivo Studies of Hydrogenated Titanium Dioxide Nanotubes with Superhydrophilic Surfaces during Early Osseointegration" Cells 11, no. 21: 3417. https://doi.org/10.3390/cells11213417
APA StyleWang, C., Gao, S., Lu, R., Wang, X., & Chen, S. (2022). In Vitro and In Vivo Studies of Hydrogenated Titanium Dioxide Nanotubes with Superhydrophilic Surfaces during Early Osseointegration. Cells, 11(21), 3417. https://doi.org/10.3390/cells11213417