Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders
Abstract
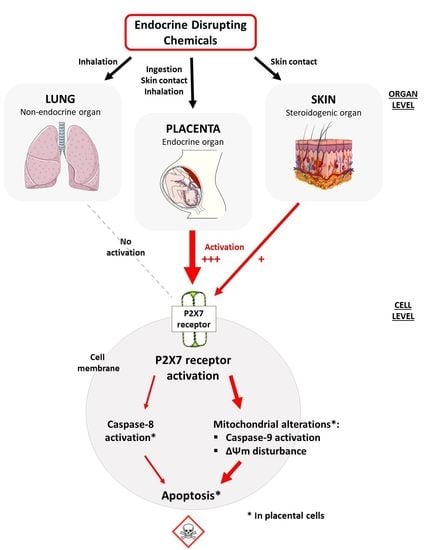
:1. Introduction
2. Materials and Methods
3. Results
3.1. Cell Viability
3.2. P2X7 Receptor Activation
3.3. EDCs Effects on Apoptosis in JEG-Tox Cells
3.3.1. Caspase-8, Caspase-9 and Caspase-3 Activity
3.3.2. Mitochondrial Membrane Potential
3.3.3. Chromatin Condensation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCS. Global Assessment of the State-of-the-Science of Endocrine Disruptor; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Paciência, I.; Cavaleiro Rufo, J.; Silva, D.; Martins, C.; Mendes, F.; Farraia, M.; Delgado, L.; de Oliveira Fernandes, E.; Padrão, P.; Moreira, P.; et al. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy 2019, 74, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Zouboulis, C.C. Endocrine-disrupting chemicals and skin manifestations. Rev. Endocr. Metab. Disord. 2016, 17, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Bamigboye, A.A.; Morris, J. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database Syst. Rev. 2003, 3, CD004353. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, M.; Xu, B.; Tang, R.; Han, X.; Qin, Y.; Xu, B.; Hang, B.; Mao, Z.; Huo, W.; et al. Parental phenols exposure and spontaneous abortion in Chinese population residing in the middle and lower reaches of the Yangtze River. Chemosphere 2013, 93, 217–222. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Garcia, J.M.; Lin, H.; Wang, Y.; Yan, P.; Wang, L.; Tan, Y.; Luo, J.; Qiu, Z.; et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS ONE 2014, 9, e87430. [Google Scholar] [CrossRef] [Green Version]
- Cantonwine, D.E.; Meeker, J.D.; Ferguson, K.K.; Mukherjee, B.; Hauser, R.; McElrath, T.F. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ. Health Perspect. 2016, 124, 1651–1655. [Google Scholar] [CrossRef]
- Etzel, T.M.; Calafat, A.M.; Ye, X.; Chen, A.; Lanphear, B.P.; Savitz, D.A.; Yolton, K.; Braun, J.M. Urinary triclosan concentrations during pregnancy and birth outcomes. Environ. Res. 2017, 156, 505–511. [Google Scholar] [CrossRef]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Calafat, A.M.; Cordero, J.F.; Meeker, J.D. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ. Health 2019, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- Mustieles, V.; Zhang, Y.; Yland, J.; Braun, J.M.; Williams, P.L.; Wylie, B.J.; Attaman, J.A.; Ford, J.B.; Azevedo, A.; Calafat, A.M.; et al. Maternal and paternal preconception exposure to phenols and preterm birth. Environ. Int. 2020, 137, 105523. [Google Scholar] [CrossRef]
- Müller, T.; Vieira, R.P.; Grimm, M.; Dürk, T.; Cicko, S.; Zeiser, R.; Jakob, T.; Martin, S.F.; Blumenthal, B.; Sorichter, S.; et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am. J. Respir. Cell Mol. Biol. 2011, 44, 456–464. [Google Scholar] [CrossRef]
- Da Silva, G.L.; Sperotto, N.D.M.; Borges, T.J.; Bonorino, C.; Takyia, C.M.; Coutinho-Silva, R.; Campos, M.M.; Zanin, R.F.; Morrone, F.B. P2X7 receptor is required for neutrophil accumulation in a mouse model of irritant contact dermatitis. Exp. Dermatol. 2013, 22, 184–188. [Google Scholar] [CrossRef]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Laprévote, O.; Rat, P. 25-Hydroxycholesterol induces both P2X7-dependent pyroptosis and caspase-dependent apoptosis in human skin model: New insights into degenerative pathways. Chem. Phys. Lipids 2017, 207, 171–178. [Google Scholar] [CrossRef]
- Tsimis, M.E.; Lei, J.; Rosenzweig, J.M.; Arif, H.; Shabi, Y.; Alshehri, W.; Talbot, C.C.; Baig-Ward, K.M.; Segars, J.; Graham, E.M.; et al. P2X7 receptor blockade prevents preterm birth and perinatal brain injury in a mouse model of intrauterine inflammation. Biol. Reprod. 2017, 97, 230–239. [Google Scholar] [CrossRef]
- Urabe, S.; Miyoshi, H.; Fujiwara, H.; Yamaoka, K.; Kudo, Y. Enhanced expression of P2X4 and P2X7 purinergic receptors in the myometrium of pregnant rats in preterm delivery models. Reprod. Sci. 2009, 16, 1186–1192. [Google Scholar] [CrossRef]
- Fodor, P.; White, B.; Khan, R. Inflammation-The role of ATP in pre-eclampsia. Microcirculation 2020, 27, e12585. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Zeng, D.; Yao, P.; Zhao, H. P2X7, a critical regulator and potential target for bone and joint diseases. J. Cell Physiol. 2019, 234, 2095–2103. [Google Scholar] [CrossRef]
- Francistiová, L.; Bianchi, C.; Di Lauro, C.; Sebastián-Serrano, Á.; de Diego-García, L.; Kobolák, J.; Dinnyés, A.; Díaz-Hernández, M. The Role of P2X7 Receptor in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 94. [Google Scholar] [CrossRef]
- Ribeiro, D.E.; Oliveira-Giacomelli, Á.; Glaser, T.; Arnaud-Sampaio, V.F.; Andrejew, R.; Dieckmann, L.; Baranova, J.; Lameu, C.; Ratajczak, M.Z.; Ulrich, H. Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol. Psychiatry 2021, 26, 1044–1059. [Google Scholar] [CrossRef]
- Mackenzie, A.B.; Young, M.T.; Adinolfi, E.; Surprenant, A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J. Biol. Chem. 2005, 280, 33968–33976. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Wang, M.; Liao, Z.; Camden, J.M.; Yu, S.; Simonyi, A.; Sun, G.Y.; Gonzalez, F.A.; Erb, L.; Seye, C.I.; et al. P2X(7) nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal. 2005, 1, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Nakatani, T.; Ohishi, A.; Okuda, H.; Higashi, Y.; Matsuo, T.; Fujimoto, S.; Nagasawa, K. Mitochondrial dysfunction is involved in P2X7 receptor-mediated neuronal cell death. J. Neurochem. 2012, 122, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.; Yerbury, J.J.; Sluyter, R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm. 2013, 2013, 271813. [Google Scholar] [CrossRef] [PubMed]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life 2021, 11, 314. [Google Scholar] [CrossRef]
- Wakx, A.; Regazzetti, A.; Dargère, D.; Auzeil, N.; Gil, S.; Evain-Brion, D.; Laprévote, O.; Rat, P. New in vitro biomarkers to detect toxicity in human placental cells: The example of benzo[A]pyrene. Toxicol. In Vitro 2016, 32, 76–85. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Androgens and ageing of the skin. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 240–245. [Google Scholar] [CrossRef]
- Roger, S.; Jelassi, B.; Couillin, I.; Pelegrin, P.; Besson, P.; Jiang, L.-H. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2584–2602. [Google Scholar] [CrossRef] [Green Version]
- Gönczi, M.; Telek, A.; Czifra, G.; Balogh, A.; Blumberg, P.M.; Bíró, T.; Csernoch, L. Altered calcium handling following the recombinant overexpression of protein kinase C isoforms in HaCaT cells. Exp. Dermatol. 2008, 17, 584–591. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, R. Placental Toxicity. In Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 68; pp. 1301–1325. [Google Scholar]
- Gingrich, J.; Ticiani, E.; Veiga-Lopez, A. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol. Metab. 2020, 31, 508–524. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, B.; He, Y.; Hong, D.; Song, S.; Huang, Y.; Zhang, T. Triclosan and triclocarbon in maternal-fetal serum, urine, and amniotic fluid samples and their implication for prenatal exposure. Environ. Pollut. 2020, 266, 115117. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Molina-Molina, J.M.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Real, M.; Sáenz, J.M.; Fernández, M.F.; Olea, N. Simultaneous determination of the UV-filters benzyl salicylate, phenyl salicylate, octyl salicylate, homosalate, 3-(4-methylbenzylidene) camphor and 3-benzylidene camphor in human placental tissue by LC-MS/MS. Assessment of their in vitro endocrine activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 936, 80–87. [Google Scholar] [CrossRef]
- Dutot, M.; Olivier, E.; Fouyet, S.; Magny, R.; Hammad, K.; Roulland, E.; Rat, P.; Fagon, R. In Vitro Chemopreventive Potential of Phlorotannins-Rich Extract from Brown Algae by Inhibition of Benzo[a]pyrene-Induced P2X7 Activation and Toxic Effects. Mar. Drugs 2021, 19, 34. [Google Scholar] [CrossRef]
- Olivier, E.; Wakx, A.; Fouyet, S.; Dutot, M.; Rat, P. JEG-3 placental cells in toxicology studies: A promising tool to reveal pregnancy disorders. Anat. Cell Biol. 2020, 54, 83–92. [Google Scholar] [CrossRef]
- Ihde, E.S.; Zamudio, S.; Loh, J.M.; Zhu, Y.; Woytanowski, J.; Rosen, L.; Liu, M.; Buckley, B. Application of a Novel Mass Spectrometric (MS) Method to Examine Exposure to Bisphenol-A and Common Substitutes in a Maternal Fetal Cohort. Hum. Ecol. Risk Assess 2018, 24, 331–346. [Google Scholar] [CrossRef]
- Tan, B.L.L.; Ali Mohd, M. Analysis of Selected Pesticides and Alkylphenols in Human Cord Blood by Gas Chromatograph-Mass Spectrometer. Talanta 2003, 61, 385–391. [Google Scholar] [CrossRef]
- Rat, P.; Olivier, E.; Tanter, C.; Wakx, A.; Dutot, M. A fast and reproducible cell- and 96-well plate-based method for the evaluation of P2X7 receptor activation using YO-PRO-1 fluorescent dye. J. Biol. Methods 2017, 4, e64. [Google Scholar] [CrossRef] [Green Version]
- Duprez, L.; Wirawan, E.; Berghe, T.V.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef]
- Zheng, L.M.; Zychlinsky, A.; Liu, C.C.; Ojcius, D.M.; Young, J.D. Extracellular ATP as a trigger for apoptosis or programmed cell death. J. Cell Biol. 1991, 112, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.N.; Smith, S.C.; To, K.F.; Sahota, D.S.; Baker, P.N. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 2001, 184, 1249–1250. [Google Scholar] [CrossRef]
- Minas, V.; Jeschke, U.; Kalantaridou, S.N.; Richter, D.U.; Reimer, T.; Mylonas, I.; Friese, K.; Makrigiannakis, A. Abortion is associated with increased expression of FasL in decidual leukocytes and apoptosis of extravillous trophoblasts: A role for CRH and urocortin. Mol. Hum. Reprod. 2007, 13, 663–673. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Saccone, G.; Migliorini, S.; Maruotti, G.M.; Esposito, G.; Mollo, A.; Martinelli, P.; Zullo, F.; D’Armiento, M. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early-onset preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Janmohamed, A.; Dolphin, C.T.; Phillips, I.R.; Shephard, E.A. Quantification and cellular localization of expression in human skin of genes encoding flavin-containing monooxygenases and cytochromes P450. Biochem. Pharmacol. 2001, 62, 777–786. [Google Scholar] [CrossRef]
- Troisi, R.; Hyer, M.; Hatch, E.E.; Titus-Ernstoff, L.; Palmer, J.R.; Strohsnitter, W.C.; Herbst, A.L.; Adam, E.; Hoover, R.N. Medical conditions among adult offspring prenatally exposed to diethylstilbestrol. Epidemiology 2013, 24, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Heude, B.; Botton, J.; Alfaidy, N.; Calafat, A.M.; Slama, R. EDEN Mother–Child Cohort Study Group Prenatal Exposure to Select Phthalates and Phenols and Associations with Fetal and Placental Weight among Male Births in the EDEN Cohort (France). Environ. Health Perspect. 2019, 127, 17002. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid. Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 2015, 103, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Hoyer, S.; Yu, R.; Soltani, K.; Lorincz, A.L.; Hiipakka, R.A.; Liao, S. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J. Investig. Dermatol. 1993, 100, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, F.; Li, C.; Otkur, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J. Photochem. PhotoBiol. B 2021, 216, 112147. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Liu, L.; Hou, X.; Jiang, J.; Wei, X.; Hao, W. Effects of bisphenol A on gap junctions in HaCaT cells as mediated by the estrogen receptor pathway. J. Appl. Toxicol. 2019, 39, 271–281. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Ferrari, D.; Los, M.; Bauer, M.K.; Vandenabeele, P.; Wesselborg, S.; Schulze-Osthoff, K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999, 447, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.; Shoji, K.F.; Sáez, J.C.; Henríquez, M.; Quest, A.F.G. FasL-triggered death of Jurkat cells requires caspase 8-induced, ATP-dependent cross-talk between Fas and the purinergic receptor P2X(7). J. Cell Physiol. 2013, 228, 485–493. [Google Scholar] [CrossRef]
- Hajra, K.M.; Liu, J.R. Apoptosome dysfunction in human cancer. Apoptosis 2004, 9, 691–704. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Feng, Y.-H.; Li, X.; Zeng, R.; Gorodeski, G.I. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am. J. Physiol Cell Physiol 2004, 287, C1349–C1358. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Tantibhedhyangkul, J.; Hawkins, K.C.; Dai, Q.; Mu, K.; Dunn, C.N.; Miller, S.E.; Price, T.M. Expression of a mitochondrial progesterone receptor in human spermatozoa correlates with a progestin-dependent increase in mitochondrial membrane potential. Andrology 2014, 2, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Provost, M.P.; Raburn, D.J.; Price, T.M. Progesterone Increases Mitochondria Membrane Potential in Non-human Primate Oocytes and Embryos. Reprod. Sci. 2020, 27, 1206–1214. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef]
- Padmini, E.; Lavanya, S.; Uthra, V. Preeclamptic placental stress and over expression of mitochondrial HSP70. Clin. Chem Lab. Med. 2009, 47, 1073–1080. [Google Scholar] [CrossRef]










| Chemical Family | Chemicals Substances | Cytotoxicity | P2X7 Activation | Caspase-8 Activity | Caspase-9 Activity | Caspase-3 Activity | Mitochondrial Membrane Potential | Chromatin Condensation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HaCaT Skin Cells | A549 Pulmonary Cells | JEG-Tox Placental Cells | HaCaT Skin Cells | A549 Pulmonary Cells | JEG-Tox Placental Cells | JEG-Tox Placental Cells | ||||||
| Bisphenols | Bisphenol A | / | No | No | / | No | Yes | No | No | No | Yes | No |
| Diethylstilbestrol | / | / | Yes | / | / | Yes | Yes | Yes | Yes | Yes | Yes | |
| Alkylphenols | 4-tert-amylphenol | / | / | No | / | / | Yes | No | Yes | No | Yes | No |
| 4-heptylphenol | / | / | Yes | / | / | No | Yes | Yes | Yes | Yes | No | |
| Chlorophenol derivatives | Triclosan | / | / | Yes | / | / | Yes | No | Yes | No | No | No |
| Parabens | Propylparaben | No | / | No | No | / | Yes | Yes | Yes | Yes | No | Yes |
| Phthalates | Benzyl butyl phthalate | / | No | Yes | / | No | Yes | Yes | Yes | No | Yes | Yes |
| Di(2-éthylhexyle) phthalate DEHP | / | / | Yes | / | / | Yes | No | Yes | No | Yes | No | |
| Camphor derivatives | 3-benzylidene camphor | No | / | Yes | Yes | / | Yes | No | No | Yes | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells 2022, 11, 495. https://doi.org/10.3390/cells11030495
Fouyet S, Olivier E, Leproux P, Dutot M, Rat P. Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells. 2022; 11(3):495. https://doi.org/10.3390/cells11030495
Chicago/Turabian StyleFouyet, Sophie, Elodie Olivier, Pascale Leproux, Mélody Dutot, and Patrice Rat. 2022. "Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders" Cells 11, no. 3: 495. https://doi.org/10.3390/cells11030495
APA StyleFouyet, S., Olivier, E., Leproux, P., Dutot, M., & Rat, P. (2022). Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells, 11(3), 495. https://doi.org/10.3390/cells11030495