How to Make Sense out of 75,000 Mesenchymal Stromal Cell Publications?
Abstract
:1. Introduction
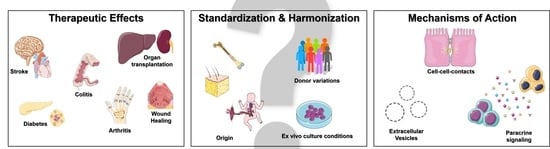
2. Therapeutic Effects of Mesenchymal Stromal Cells
3. Standardization and Harmonization of MSC Therapy
4. Mechanisms of Action of Mesenchymal Stromal Cells
5. How Should We Proceed?
6. Where Will MSC Research Eventually Lead to?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in Transplants of Bone Marrow Cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning in Vitro and Retransplantation in Vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. Npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, H.M.; Koc, O.N.; Devine, S.M.; Curtin, P.; Maziarz, R.T.; Holland, H.K.; Shpall, E.J.; McCarthy, P.; Atkinson, K.; Cooper, B.W.; et al. Cotransplantation of HLA-Identical Sibling Culture-Expanded Mesenchymal Stem Cells and Hematopoietic Stem Cells in Hematologic Malignancy Patients. Biol. Blood Marrow Transplant. 2005, 11, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, H.M.; Haynesworth, S.E.; Gerson, S.L.; Rosenthal, N.S.; Caplan, A.I. Ex Vivo Expansion and Subsequent Infusion of Human Bone Marrow-Derived Stromal Progenitor Cells (Mesenchymal Progenitor Cells): Implications for Therapeutic Use. Bone Marrow Transplant. 1995, 16, 557–564. [Google Scholar]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. Autologous Mesenchymal Stem/Stromal Cells in Their Medication Practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Sun, L.Y.; Zhang, H.Y.; Feng, X.B.; Hou, Y.Y.; Lu, L.W.; Fan, L.M. Abnormality of Bone Marrow-Derived Mesenchymal Stem Cells in Patients with Systemic Lupus Erythematosus. Lupus 2007, 16, 121–128. [Google Scholar] [CrossRef]
- Larghero, J.; Farge, D.; Braccini, A.; Lecourt, S.; Scherberich, A.; Foïs, E.; Verrecchia, F.; Daikeler, T.; Gluckman, E.; Tyndall, A.; et al. Phenotypical and Functional Characteristics of in Vitro Expanded Bone Marrow Mesenchymal Stem Cells from Patients with Systemic Sclerosis. Ann. Rheum. Dis. 2008, 67, 443–449. [Google Scholar] [CrossRef]
- Capelli, C.; Zaccara, E.; Cipriani, P.; Di Benedetto, P.; Maglione, W.; Andracco, R.; Di Luca, G.; Pignataro, F.; Giacomelli, R.; Introna, M.; et al. Phenotypical and Functional Characteristics of In Vitro-Expanded Adipose-Derived Mesenchymal Stromal Cells From Patients With Systematic Sclerosis. Cell Transplant. 2017, 26, 841–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinders, M.E.J.; Roemeling-van Rhijn, M.; Khairoun, M.; Lievers, E.; de Vries, D.K.; Schaapherder, A.F.M.; Wong, S.W.S.; Zwaginga, J.J.; Duijs, J.M.; van Zonneveld, A.J.; et al. Bone Marrow-Derived Mesenchymal Stromal Cells from Patients with End-Stage Renal Disease Are Suitable for Autologous Therapy. Cytotherapy 2013, 15, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.-J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated Intra-Articular Injection of Allogeneic Mesenchymal Stem Cells Causes an Adverse Response Compared to Autologous Cells in the Equine Model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.-P.; Sun, Z.; Miyagi, Y.; McDonald Kinkaid, H.; Zhang, L.; Weisel, R.D.; Li, R.-K. Differentiation of Allogeneic Mesenchymal Stem Cells Induces Immunogenicity and Limits Their Long-Term Benefits for Myocardial Repair. Circulation 2010, 122, 2419–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of Allogeneic vs Autologous Bone Marrow–Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients with Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef]
- Thakkar, U.G.; Trivedi, H.L.; Vanikar, A.V.; Dave, S.D. Insulin-Secreting Adipose-Derived Mesenchymal Stromal Cells with Bone Marrow-Derived Hematopoietic Stem Cells from Autologous and Allogenic Sources for Type 1 Diabetes Mellitus. Cytotherapy 2015, 17, 940–947. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Tolar, J.; Villeneuve, P.; Keating, A. Mesenchymal Stromal Cells for Graft-Versus-Host Disease. Hum. Gene Ther. 2011, 22, 257–262. [Google Scholar] [CrossRef]
- Reinders, M.E.J.; van Kooten, C.; Rabelink, T.J.; de Fijter, J.W. Mesenchymal Stromal Cell Therapy for Solid Organ Transplantation. Transplantation 2018, 102, 35–43. [Google Scholar] [CrossRef]
- Vandermeulen, M.; Erpicum, P.; Weekers, L.; Briquet, A.; Lechanteur, C.; Detry, O.; Beguin, Y.; Jouret, F. Mesenchymal Stromal Cells in Solid Organ Transplantation. Transplantation 2020, 104, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Päth, G.; Perakakis, N.; Mantzoros, C.S.; Seufert, J. Stem Cells in the Treatment of Diabetes Mellitus—Focus on Mesenchymal Stem Cells. Metabolism 2019, 90, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuascut, F.X.; Hutton, G.J. Stem Cell-Based Therapies for Multiple Sclerosis: Current Perspectives. Biomedicines 2019, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.A.; Lapa E Silva, J.R.; Rocco, P.R. Mesenchymal Stromal Cell Therapy in COPD: From Bench to Bedside. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3017–3027. [Google Scholar] [CrossRef] [Green Version]
- Chinnadurai, R.; Ng, S.; Velu, V.; Galipeau, J. Challenges in Animal Modelling of Mesenchymal Stromal Cell Therapy for Inflammatory Bowel Disease. World J. Gastroenterol. 2015, 21, 4779–4787. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.-X.; Sun, L.; Wallis, C.U.; Zhou, J.-S.; Ao, L.-J.; Li, Q.; Sham, P.C. Rational Use of Mesenchymal Stem Cells in the Treatment of Autism Spectrum Disorders. World J. Stem Cells 2019, 11, 55–72. [Google Scholar] [CrossRef]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e20. [Google Scholar] [CrossRef]
- Banerjee, A.; Bizzaro, D.; Burra, P.; Di Liddo, R.; Pathak, S.; Arcidiacono, D.; Cappon, A.; Bo, P.; Conconi, M.T.; Crescenzi, M.; et al. Umbilical Cord Mesenchymal Stem Cells Modulate Dextran Sulfate Sodium Induced Acute Colitis in Immunodeficient Mice. Stem Cell Res. Ther. 2015, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous Bone Marrow–Derived Cultured Mesenchymal Stem Cells Delivered in a Fibrin Spray Accelerate Healing in Murine and Human Cutaneous Wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic Delivery of Bone Marrow-Derived Mesenchymal Stem Cells to the Infarcted Myocardium: Feasibility, Cell Migration, and Body Distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, P.R.; Greeley, M.; Halloran, S.M.; MacDonald, K.A.; Kittleson, M.D. Intra-Coronary Arterial Injection of Mesenchymal Stromal Cells and Microinfarction in Dogs. Lancet 2004, 363, 783–784. [Google Scholar] [CrossRef]
- Choi, J.-J.; Yoo, S.-A.; Park, S.-J.; Kang, Y.-J.; Kim, W.-U.; Oh, I.-H.; Cho, C.-S. Mesenchymal Stem Cells Overexpressing Interleukin-10 Attenuate Collagen-Induced Arthritis in Mice. Clin. Exp. Immunol. 2008, 153, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Rota, C.; Breno, M.; Mele, C.; Noris, M.; Introna, M.; Capelli, C.; Longaretti, L.; Rottoli, D.; et al. Human Mesenchymal Stromal Cells Transplanted into Mice Stimulate Renal Tubular Cells and Enhance Mitochondrial Function. Nat. Commun. 2017, 8, 983. [Google Scholar] [CrossRef]
- Casiraghi, F.; Azzollini, N.; Todeschini, M.; Cavinato, R.A.; Cassis, P.; Solini, S.; Rota, C.; Morigi, M.; Introna, M.; Maranta, R.; et al. Localization of Mesenchymal Stromal Cells Dictates Their Immune or Proinflammatory Effects in Kidney Transplantation. Am. J. Transplant. 2012, 12, 2373–2383. [Google Scholar] [CrossRef]
- De Martino, M.; Zonta, S.; Rampino, T.; Gregorini, M.; Frassoni, F.; Piotti, G.; Bedino, G.; Cobianchi, L.; Dal Canton, A.; Dionigi, P.; et al. Mesenchymal Stem Cells Infusion Prevents Acute Cellular Rejection in Rat Kidney Transplantation. Transplant. Proc. 2010, 42, 1331–1335. [Google Scholar] [CrossRef]
- Hara, Y.; Stolk, M.; Ringe, J.; Dehne, T.; Ladhoff, J.; Kotsch, K.; Reutzel-Selke, A.; Reinke, P.; Volk, H.-D.; Seifert, M. In Vivo Effect of Bone Marrow-Derived Mesenchymal Stem Cells in a Rat Kidney Transplantation Model with Prolonged Cold Ischemia. Transpl. Int. 2011, 24, 1112–1123. [Google Scholar] [CrossRef]
- McIntyre, L.A.; Moher, D.; Fergusson, D.A.; Sullivan, K.J.; Mei, S.H.J.; Lalu, M.; Marshall, J.; Mcleod, M.; Griffin, G.; Grimshaw, J.; et al. Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review. PLoS ONE 2016, 11, e0147170. [Google Scholar] [CrossRef] [Green Version]
- Fengyun, W.; LiXin, Z.; Xinhua, Q.; Bin, F. Mesenchymal Stromal Cells Attenuate Infection-Induced Acute Respiratory Distress Syndrome in Animal Experiments: A Meta-Analysis. Cell Transplant. 2020, 29, 963689720969186. [Google Scholar] [CrossRef]
- Riecke, J.; Johns, K.M.; Cai, C.; Vahidy, F.S.; Parsha, K.; Furr-Stimming, E.; Schiess, M.; Savitz, S.I. A Meta-Analysis of Mesenchymal Stem Cells in Animal Models of Parkinson’s Disease. Stem Cells Dev. 2015, 24, 2082–2090. [Google Scholar] [CrossRef]
- Vu, Q.; Xie, K.; Eckert, M.; Zhao, W.; Cramer, S.C. Meta-Analysis of Preclinical Studies of Mesenchymal Stromal Cells for Ischemic Stroke. Neurology 2014, 82, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Kanelidis, A.J.; Premer, C.; Lopez, J.; Balkan, W.; Hare, J.M. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction. Circ. Res. 2017, 120, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Lombardo, E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl. Med. 2019, 8, 1126–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.; McKenna, D.H.; Rocco, P.R.M.; Weiss, D.J. Freshly Thawed and Continuously Cultured Human Bone Marrow-Derived Mesenchymal Stromal Cells Comparably Ameliorate Allergic Airways Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015, 4, 615–624. [Google Scholar] [CrossRef]
- Gramlich, O.W.; Burand, A.J.; Brown, A.J.; Deutsch, R.J.; Kuehn, M.H.; Ankrum, J.A. Cryopreserved Mesenchymal Stromal Cells Maintain Potency in a Retinal Ischemia/Reperfusion Injury Model: Toward an off-the-Shelf Therapy. Sci. Rep. 2016, 6, 26463. [Google Scholar] [CrossRef]
- Reinders, M.E.J.; Dreyer, G.J.; Bank, J.R.; Roelofs, H.; Heidt, S.; Roelen, D.L.; Zandvliet, M.L.; Huurman, V.A.L.; Fibbe, W.E.; van Kooten, C.; et al. Safety of Allogeneic Bone Marrow Derived Mesenchymal Stromal Cell Therapy in Renal Transplant Recipients: The Neptune Study. J. Transl. Med. 2015, 13, 344. [Google Scholar] [CrossRef] [Green Version]
- Perico, N.; Casiraghi, F.; Introna, M.; Gotti, E.; Todeschini, M.; Cavinato, R.A.; Capelli, C.; Rambaldi, A.; Cassis, P.; Rizzo, P.; et al. Autologous Mesenchymal Stromal Cells and Kidney Transplantation: A Pilot Study of Safety and Clinical Feasibility. Clin. J. Am. Soc. Nephrol. 2011, 6, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Erpicum, P.; Weekers, L.; Detry, O.; Bonvoisin, C.; Delbouille, M.-H.; Grégoire, C.; Baudoux, E.; Briquet, A.; Lechanteur, C.; Maggipinto, G.; et al. Infusion of Third-Party Mesenchymal Stromal Cells after Kidney Transplantation: A Phase I-II, Open-Label, Clinical Study. Kidney Int. 2019, 95, 693–707. [Google Scholar] [CrossRef]
- Soeder, Y.; Loss, M.; Johnson, C.L.; Hutchinson, J.A.; Haarer, J.; Ahrens, N.; Offner, R.; Deans, R.J.; Van Bokkelen, G.; Geissler, E.K.; et al. First-in-Human Case Study: Multipotent Adult Progenitor Cells for Immunomodulation After Liver Transplantation. Stem Cells Transl. Med. 2015, 4, 899–904. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Z.; Wang, Y.; Xu, R.; Sun, Y.; Zhang, M.; Yu, X.; Wang, H.; Meng, L.; Su, H.; et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl. Med. 2017, 6, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Detry, O.; Vandermeulen, M.; Delbouille, M.-H.; Somja, J.; Bletard, N.; Briquet, A.; Lechanteur, C.; Giet, O.; Baudoux, E.; Hannon, M.; et al. Infusion of Mesenchymal Stromal Cells after Deceased Liver Transplantation: A Phase I-II, Open-Label, Clinical Study. J. Hepatol. 2017, 67, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal Stem (Stromal) Cells for Treatment of ARDS: A Phase 1 Clinical Trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Weiss, D.J.; Casaburi, R.; Flannery, R.; LeRoux-Williams, M.; Tashkin, D.P. A Placebo-Controlled, Randomized Trial of Mesenchymal Stem Cells in COPD. Chest 2013, 143, 1590–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with Allogeneic Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome (START Study): A Randomised Phase 2a Safety Trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- de Oliveira, H.G.; Cruz, F.F.; Antunes, M.A.; de Macedo Neto, A.V.; Oliveira, G.A.; Svartman, F.M.; Borgonovo, T.; Rebelatto, C.L.K.; Weiss, D.J.; Brofman, P.R.S.; et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Transl. Med. 2017, 6, 962–969. [Google Scholar] [CrossRef]
- Stolk, J.; Broekman, W.; Mauad, T.; Zwaginga, J.J.; Roelofs, H.; Fibbe, W.E.; Oostendorp, J.; Bajema, I.; Versteegh, M.I.M.; Taube, C.; et al. A Phase I Study for Intravenous Autologous Mesenchymal Stromal Cell Administration to Patients with Severe Emphysema. QJM 2016, 109, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Chambers, D.C.; Enever, D.; Lawrence, S.; Sturm, M.J.; Herrmann, R.; Yerkovich, S.; Musk, M.; Hopkins, P.M.A. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl. Med. 2017, 6, 1152–1157. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Ramcharan, R.N.; Friend, P.J.; Vaidya, A. Mesenchymal Stromal Cells Promote Bowel Regeneration after Intestinal Transplantation: Myth to Mucosa. Transpl. Int. 2013, 26, e91–e93. [Google Scholar] [CrossRef]
- Doğan, S.M.; Kılınç, S.; Kebapçı, E.; Tuğmen, C.; Gürkan, A.; Baran, M.; Kurtulmuş, Y.; Olmez, M.; Karaca, C. Mesenchymal Stem Cell Therapy in Patients with Small Bowel Transplantation: Single Center Experience. World J. Gastroenterol. 2014, 20, 8215–8220. [Google Scholar] [CrossRef]
- Barnhoorn, M.C.; Wasser, M.N.J.M.; Roelofs, H.; Maljaars, P.W.J.; Molendijk, I.; Bonsing, B.A.; Oosten, L.E.M.; Dijkstra, G.; van der Woude, C.J.; Roelen, D.L.; et al. Long-Term Evaluation of Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cell Therapy for Crohn’s Disease Perianal Fistulas. J. Crohns Colitis 2020, 14, 64–70. [Google Scholar] [CrossRef]
- Kebriaei, P.; Hayes, J.; Daly, A.; Uberti, J.; Marks, D.I.; Soiffer, R.; Waller, E.K.; Burke, E.; Skerrett, D.; Shpall, E.; et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded Allogeneic Adipose-Derived Mesenchymal Stem Cells (Cx601) for Complex Perianal Fistulas in Crohn’s Disease: A Phase 3 Randomised, Double-Blind Controlled Trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; D Hoore, A.J.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panés, J. Follow-up Study to Evaluate the Long-Term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients with Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis. Colon Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Kurtzberg, J.; Abdel-Azim, H.; Carpenter, P.; Chaudhury, S.; Horn, B.; Mahadeo, K.; Nemecek, E.; Neudorf, S.; Prasad, V.; Prockop, S.; et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Sahu, K.K.; Siddiqui, A.D.; Cerny, J. Mesenchymal Stem Cells in COVID-19: A Journey from Bench to Bedside. Lab. Med. 2021, 52, 24–35. [Google Scholar] [CrossRef]
- Adas, G.; Cukurova, Z.; Yasar, K.K.; Yilmaz, R.; Isiksacan, N.; Kasapoglu, P.; Yesilbag, Z.; Koyuncu, I.D.; Karaoz, E. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled Trial. Cell Transplant. 2021, 30, 9636897211024942. [Google Scholar] [CrossRef]
- Saleh, M.; Vaezi, A.A.; Aliannejad, R.; Sohrabpour, A.A.; Kiaei, S.Z.F.; Shadnoush, M.; Siavashi, V.; Aghaghazvini, L.; Khoundabi, B.; Abdoli, S.; et al. Cell Therapy in Patients with COVID-19 Using Wharton’s Jelly Mesenchymal Stem Cells: A Phase 1 Clinical Trial. Stem Cell Res. Ther. 2021, 12, 410. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Dilogo, I.H.; Aditianingsih, D.; Sugiarto, A.; Burhan, E.; Damayanti, T.; Sitompul, P.A.; Mariana, N.; Antarianto, R.D.; Liem, I.K.; Kispa, T.; et al. Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 1279–1287. [Google Scholar] [CrossRef]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T.; Shi, L.; Fu, J.; Jiang, T.; et al. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy in Patients with COVID-19: A Phase 1 Clinical Trial. Signal Transduct. Target. Ther. 2020, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perico, N.; Casiraghi, F.; Gotti, E.; Introna, M.; Todeschini, M.; Cavinato, R.A.; Capelli, C.; Rambaldi, A.; Cassis, P.; Rizzo, P.; et al. Mesenchymal Stromal Cells and Kidney Transplantation: Pretransplant Infusion Protects from Graft Dysfunction While Fostering Immunoregulation. Transpl. Int. 2013, 26, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, H.; Weiss, D.J.; Rolandsson Enes, S.; Laffey, J.G.; English, K. The Inflammatory Lung Microenvironment; a Key Mediator in MSC Licensing. Cells 2021, 10, 2982. [Google Scholar] [CrossRef]
- Leuning, D.G.; Beijer, N.R.M.; du Fossé, N.A.; Vermeulen, S.; Lievers, E.; van Kooten, C.; Rabelink, T.J.; de Boer, J. The Cytokine Secretion Profile of Mesenchymal Stromal Cells Is Determined by Surface Structure of the Microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef]
- Wong, S.W.; Lenzini, S.; Giovanni, R.; Knowles, K.; Shin, J.-W. Matrix Biophysical Cues Direct Mesenchymal Stromal Cell Functions in Immunity. Acta Biomater. 2021, 133, 126–138. [Google Scholar] [CrossRef]
- Moll, G.; Hoogduijn, M.J.; Ankrum, J.A. Safety, Efficacy and Mechanisms of Action of Mesenchymal Stem Cell Therapies. Front. Immunol. 2020, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Calcat-I-Cervera, S.; Sanz-Nogués, C.; O’Brien, T. When Origin Matters: Properties of Mesenchymal Stromal Cells from Different Sources for Clinical Translation in Kidney Disease. Front. Med. 2021, 8, 728496. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Kocaömer, A.; Ferlik, K.; Bugert, P. Comparing Mesenchymal Stromal Cells from Different Human Tissues: Bone Marrow, Adipose Tissue and Umbilical Cord Blood. Biomed. Mater. Eng. 2008, 18, S71–S76. [Google Scholar] [PubMed]
- Valencia, J.; Blanco, B.; Yáñez, R.; Vázquez, M.; Herrero Sánchez, C.; Fernández-García, M.; Rodríguez Serrano, C.; Pescador, D.; Blanco, J.F.; Hernando-Rodríguez, M.; et al. Comparative Analysis of the Immunomodulatory Capacities of Human Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stromal Cells from the Same Donor. Cytotherapy 2016, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, S.A.; Mankani, M.H.; Bianco, P.; Robey, P.G. Enumeration of the Colony-Forming Units–Fibroblast from Mouse and Human Bone Marrow in Normal and Pathological Conditions. Stem Cell Res. 2009, 2, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-Related Changes in Human Bone Marrow-Derived Mesenchymal Stem Cells: Consequences for Cell Therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor Variation in the Growth Properties and Osteogenic Potential of Human Marrow Stromal Cells. J. Cell Biochem. 1999, 75, 424–436. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Krüger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Pasumarthy, K.K.; Doni Jayavelu, N.; Kilpinen, L.; Andrus, C.; Battle, S.L.; Korhonen, M.; Lehenkari, P.; Lund, R.; Laitinen, S.; Hawkins, R.D. Methylome Analysis of Human Bone Marrow MSCs Reveals Extensive Age- and Culture-Induced Changes at Distal Regulatory Elements. Stem Cell Rep. 2017, 9, 999–1015. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging in Vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Wiese, D.M.; Ruttan, C.C.; Wood, C.A.; Ford, B.N.; Braid, L.R. Accumulating Transcriptome Drift Precedes Cell Aging in Human Umbilical Cord-Derived Mesenchymal Stromal Cells Serially Cultured to Replicative Senescence. Stem Cells Transl. Med. 2019, 8, 945–958. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.; Hodgson-Garms, M.; Frith, J.E.; Genever, P. Multiplicity of Mesenchymal Stromal Cells: Finding the Right Route to Therapy. Front. Immunol. 2019, 10, 1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal Stem Cells Are Short-Lived and Do Not Migrate beyond the Lungs after Intravenous Infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-Conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal Stem/Stromal Cells as a Pharmacological and Therapeutic Approach to Accelerate Angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine Action Accounts for Marked Protection of Ischemic Heart by Akt-Modified Mesenchymal Stem Cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Ragni, E.; Papait, A.; Perucca Orfei, C.; Silini, A.R.; Colombini, A.; Viganò, M.; Libonati, F.; Parolini, O.; de Girolamo, L. Amniotic Membrane-Mesenchymal Stromal Cells Secreted Factors and Extracellular Vesicle-MiRNAs: Anti-Inflammatory and Regenerative Features for Musculoskeletal Tissues. Stem Cells Transl. Med. 2021, 10, 1044–1062. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.R.R.; Lee, O.; Eggenhofer, E.; Geissler, E.; Korevaar, S.S.; Soeder, Y.; Schlitt, H.J.; Geissler, E.K.; Hoogduijn, M.J.; Dahlke, M.H. Differential Effects of Heat-Inactivated, Secretome-Deficient MSC and Metabolically Active MSC in Sepsis and Allogenic Heart Transplantation. Stem Cells 2020, 38, 797–807. [Google Scholar] [CrossRef]
- da C. Gonçalves, F.; Luk, F.; Korevaar, S.S.; Bouzid, R.; Paz, A.H.; López-Iglesias, C.; Baan, C.C.; Merino, A.; Hoogduijn, M.J. Membrane Particles Generated from Mesenchymal Stromal Cells Modulate Immune Responses by Selective Targeting of Pro-Inflammatory Monocytes. Sci. Rep. 2017, 7, 12100. [Google Scholar] [CrossRef]
- Luk, F.; de Witte, S.F.H.; Korevaar, S.S.; Roemeling-van Rhijn, M.; Franquesa, M.; Strini, T.; van den Engel, S.; Gargesha, M.; Roy, D.; Dor, F.J.M.F.; et al. Inactivated Mesenchymal Stem Cells Maintain Immunomodulatory Capacity. Stem Cells Dev. 2016, 25, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.M.; Gremmels, H.; Giebel, B.; Lim, S.K. Proteomic Signature of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles. Proteomics 2019, 19, e1800163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, S.; Deregibus, M.C.; Camussi, G. The Secretome of Mesenchymal Stromal Cells: Role of Extracellular Vesicles in Immunomodulation. Immunol. Lett. 2015, 168, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal Stem Cell-Derived Microvesicles Protect against Acute Tubular Injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [Green Version]
- Anger, F.; Camara, M.; Ellinger, E.; Germer, C.-T.; Schlegel, N.; Otto, C.; Klein, I. Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Improve Liver Regeneration After Ischemia Reperfusion Injury in Mice. Stem Cells Dev. 2019, 28, 1451–1462. [Google Scholar] [CrossRef]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in Mesenchymal Stromal Cells Induces in Vivo Recipient-Mediated Immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [Green Version]
- de Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Cheung, T.S.; Galleu, A.; von Bonin, M.; Bornhäuser, M.; Dazzi, F. Apoptotic Mesenchymal Stromal Cells Induce Prostaglandin E2 in Monocytes: Implications for the Monitoring of Mesenchymal Stromal Cell Activity. Haematologica 2019, 104, e438–e441. [Google Scholar] [CrossRef] [Green Version]
- Bank, J.R.; Rabelink, T.J.; de Fijter, J.W.; Reinders, M.E.J. Safety and Efficacy Endpoints for Mesenchymal Stromal Cell Therapy in Renal Transplant Recipients. J. Immunol. Res. 2015, 2015, 391797. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Montserrat, N.; van der Laan, L.J.W.; Dazzi, F.; Perico, N.; Kastrup, J.; Gilbo, N.; Ploeg, R.J.; Roobrouck, V.; Casiraghi, F.; et al. The Emergence of Regenerative Medicine in Organ Transplantation: 1st European Cell Therapy and Organ Regeneration Section Meeting. Transpl. Int. 2020, 33, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, D. Negative Results Are Disappearing from Most Disciplines and Countries. Scientometrics 2012, 90, 891–904. [Google Scholar] [CrossRef]
- Mehta, D. Highlight Negative Results to Improve Science. Available online: https://www.nature.com/articles/d41586-019-02960-3 (accessed on 17 January 2022).
- Echevarría, L.; Malerba, A.; Arechavala-Gomeza, V. Researcher’s Perceptions on Publishing “Negative” Results and Open Access. Nucleic Acid Ther. 2021, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Nimpf, S.; Keays, D.A. Why (and How) We Should Publish Negative Data. EMBO Rep. 2020, 21, e49775. [Google Scholar] [CrossRef] [PubMed]
- Hartshorne, J.K.; Schachner, A. Tracking Replicability as a Method of Post-Publication Open Evaluation. Front. Comput. Neurosci. 2012, 6, 8. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mönch, D.; Reinders, M.E.J.; Dahlke, M.H.; Hoogduijn, M.J. How to Make Sense out of 75,000 Mesenchymal Stromal Cell Publications? Cells 2022, 11, 1419. https://doi.org/10.3390/cells11091419
Mönch D, Reinders MEJ, Dahlke MH, Hoogduijn MJ. How to Make Sense out of 75,000 Mesenchymal Stromal Cell Publications? Cells. 2022; 11(9):1419. https://doi.org/10.3390/cells11091419
Chicago/Turabian StyleMönch, Dina, Marlies E. J. Reinders, Marc H. Dahlke, and Martin J. Hoogduijn. 2022. "How to Make Sense out of 75,000 Mesenchymal Stromal Cell Publications?" Cells 11, no. 9: 1419. https://doi.org/10.3390/cells11091419
APA StyleMönch, D., Reinders, M. E. J., Dahlke, M. H., & Hoogduijn, M. J. (2022). How to Make Sense out of 75,000 Mesenchymal Stromal Cell Publications? Cells, 11(9), 1419. https://doi.org/10.3390/cells11091419